Overview
Mastering amperometry in pharmaceutical analysis is crucial for ensuring accurate drug evaluation. This technique necessitates a comprehensive understanding of its fundamental principles, alongside the gathering of essential equipment and the calibration of instruments. A solid grasp of amperometric methods and their diverse applications—such as drug quantification and quality control—is vital. Precise calibration practices further enhance the reliability of results in pharmaceutical testing. Therefore, it is imperative for professionals in the field to apply these techniques effectively, reinforcing the significance of high-quality scientific instruments in laboratory settings.
Introduction
In the realm of pharmaceutical analysis, amperometry emerges as a pivotal electroanalytical technique, essential for ensuring the accuracy and reliability of drug testing and quality control. This method measures the current generated during the oxidation or reduction of specific analytes, facilitating the precise quantification of active pharmaceutical ingredients. Moreover, it plays a crucial role in real-time monitoring and impurity detection, underscoring its significance in laboratory settings.
As advancements in technology continue to shape the landscape of electrochemical analysis, understanding the fundamental components and calibration processes of amperometry becomes increasingly vital for professionals in the field.
This article delves into the intricacies of amperometric techniques, highlighting their applications, necessary equipment, and best practices to enhance analytical capabilities in pharmaceutical laboratories.
Understand Amperometry Fundamentals
Amperometry is that quantifies the current generated during the oxidation or reduction of an analyte at an electrode. This method operates by applying a constant potential to a working electrode, thereby facilitating the detection of electroactive species within a solution. A comprehensive understanding of these components is vital for effectively applying amperometry in pharmaceutical analysis, as the effectiveness of this technique hinges on three essential components:
- A working electrode
- A reference electrode
- A counter electrode
These components directly impact the accuracy and reliability of measurements. Recent advancements in amperometry underscore its significance in electroanalytical chemistry, which is crucial for both current and future scientific endeavors, particularly within pharmaceutical applications. For instance, the versatility of amperometric methods—such as direct, pulsed, and chronoamperometry—empowers researchers to tailor their strategies to meet specific analytical challenges in drug testing, highlighting the critical role of amperometry in electroanalytical applications.
Industry leaders emphasize the need for reliable biosensors to assess various analytes in pharmaceuticals. Statistical evaluations comparing amperometric techniques, such as direct amperometry versus pulsed amperometry, have shown no significant differences in precision, reinforcing the reliability of these methods. Furthermore, the double layer at the electrode-solution interface plays a crucial role in affecting charge transfer and diffusion, thereby influencing electrochemical assessments. As the field continues to evolve, it will be essential for pharmaceutical professionals to remain informed about current trends and advancements in amperometry to enhance their analytical capabilities.
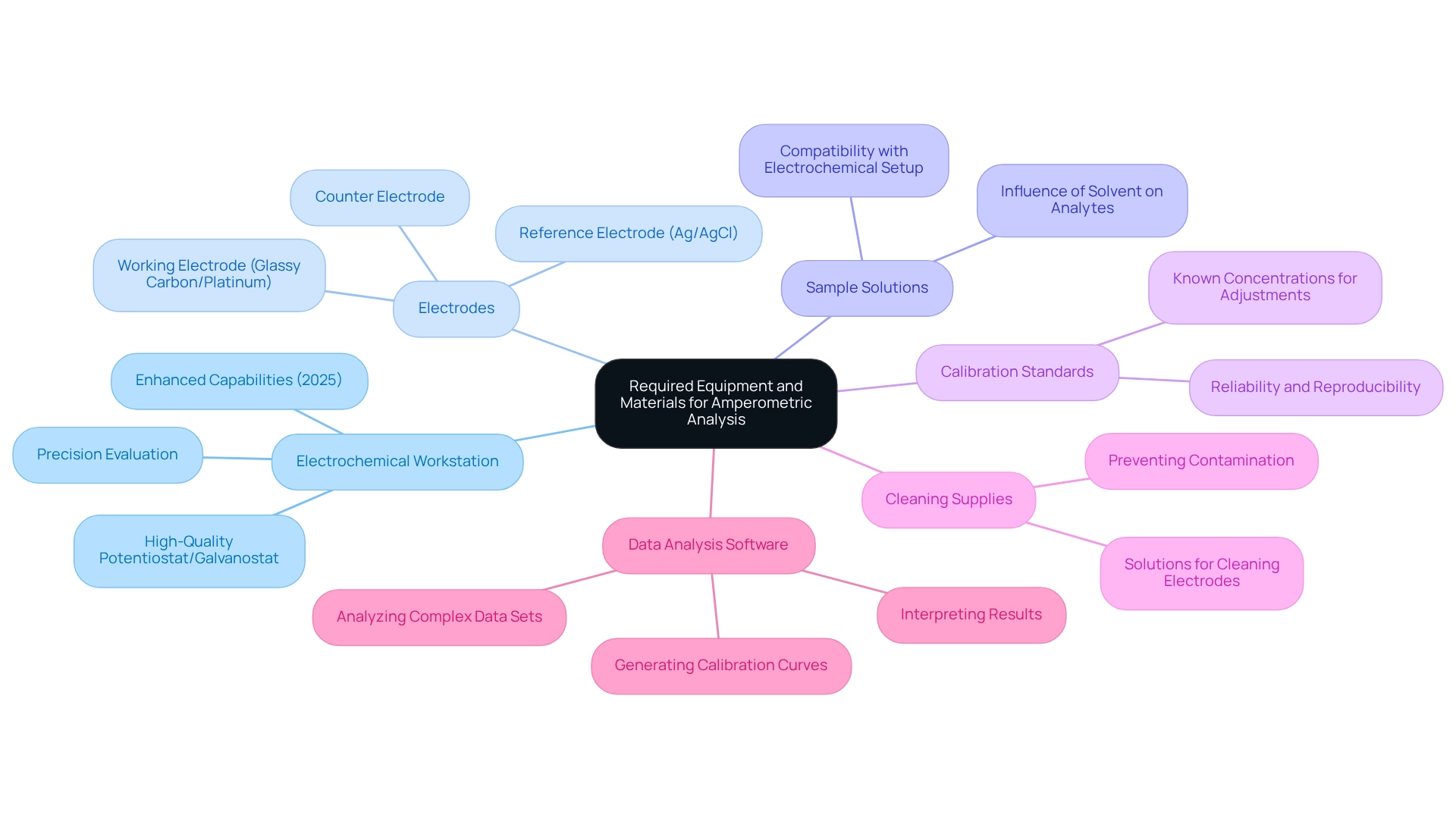
Gather Required Equipment and Materials
To conduct effective amperometric analysis in pharmaceutical laboratories, certain equipment and materials are indispensable:
- Electrochemical Workstation: A high-quality potentiostat/galvanostat is essential for controlling the potential and measuring current accurately. The latest designs in 2025 offer enhanced capabilities for precision evaluation. As Thomas J. Roussel articulates, "The applied potential must be sufficient to cause facile oxidation or reduction of the target analytes," highlighting the critical role of this equipment.
- Electrodes: Employ a suitable working electrode, such as glassy carbon or platinum, in conjunction with a reference electrode (e.g., Ag/AgCl) and a counter electrode to ensure reliable readings. Each type of electrode serves a specific function within the electrochemical arrangement, thereby enhancing the precision of the measurements.
- Sample Solutions: Prepare the analytes in appropriate solvents, ensuring compatibility with the electrochemical setup. The choice of solvent can significantly influence the electrochemical behavior of the analytes.
- Calibration Standards: Utilize known concentrations of the analyte for adjustments, which are crucial for precise quantification. This step is vital to guarantee that the results are both reliable and reproducible.
- Cleaning Supplies: Keep solutions available for cleaning electrodes to prevent contamination, which can adversely affect results. Maintaining clean electrodes is fundamental for obtaining accurate measurements.
- Data Analysis Software: Implement advanced software for interpreting results and generating calibration curves, thereby facilitating a deeper understanding of the data. This software aids in analyzing complex data sets, enhancing the overall quality of the evaluation.
Before initiating experiments, ensure that all equipment is properly calibrated and functioning. Laboratory managers stress the importance of reliable materials for amperometry and electrochemical analysis, as they directly influence the quality of results. For instance, research indicates that for the greatest dilution of Strep-HRP at 1:20 was 784 nA, underscoring the necessity for precise instruments to achieve such readings. Furthermore, recent advancements in point-of-care diagnostic tools illustrate the increasing relevance of electrochemical immunosensors in clinical applications, emphasizing the importance of investing in high-performance electrochemical workstations. The transition of point-of-care diagnostics into clinical practice further underscores the significance of these techniques in real-world applications.
Calibrate Instruments for Accurate Measurements
To achieve precise measurements in amperometric analysis, it is essential to adhere to the following calibration steps:
- Prepare Calibration Standards: Develop a series of standard solutions with known analyte concentrations to establish a reliable reference. This foundational step ensures that your measurements are anchored in accuracy.
- Set Up the Electrochemical Workstation: Connect the electrodes to the potentiostat, ensuring all settings are accurately configured for optimal performance. Proper setup is crucial for obtaining valid results.
- Run Calibration Measurements: Measure the current response for each standard solution at a fixed potential, meticulously recording the current values for analysis. This data collection is vital for creating a reliable standard curve.
- Generate Standard Curve: Plot the recorded current responses against the known concentrations to create a standard curve, which is essential for determining sample concentrations accurately. A well-constructed curve is the backbone of precise analysis.
- Validate Adjustment: Utilize extra standards to verify the accuracy of the adjustment curve, making modifications as needed to uphold precision. Continuous validation is key to maintaining the integrity of your measurements.
Routine adjustment is essential to maintain measurement precision, especially when instruments are employed over prolonged durations. Statistics suggest that upholding a bias criterion of 0.33*TEa can greatly reduce random error, highlighting the significance of regular adjustment practices. Moreover, and calculators are accessible to aid laboratories in method validation and quality assurance, offering additional resources for effective adjustment. Case studies, such as those investigating adjustment curves in spectroscopy, emphasize how methods including amperometry enhance analytical skills across diverse applications, reinforcing the importance of thorough standardization in electrochemical measurements. As noted by David Coleman, an applied statistician, "it is not possible to generate a truly representative prediction interval, thereby precluding a sound assessment of the uncertainty associated with ," underscoring the critical role of calibration in ensuring reliable analytical results.
Apply Amperometry in Pharmaceutical Analysis
The role of amperometry is crucial in pharmaceutical evaluation, providing a variety of applications that enhance both drug development and quality control processes. Key applications include drug quantification, quality control, in vivo monitoring, and flow injection analysis (FIA). Each of these areas underscores the importance of amperometry techniques in guaranteeing the safety and efficacy of pharmaceutical products.
Drug Quantification: Amperometry techniques are employed to accurately measure the concentration of active pharmaceutical ingredients (APIs) in formulations, ensuring that dosages meet regulatory standards. For instance, recovery for Sample 5 using HPLC-UV was reported at 101.0 ± 2.3%, demonstrating the accuracy of these methods. This precision is vital for compliance and patient safety.
Quality Control: These methods are vital for maintaining the consistency and purity of pharmaceutical products. By detecting impurities or degradation products, amperometry plays a crucial role in safeguarding product integrity, which is essential in a market that demands high-quality standards.
In Vivo Monitoring: Amperometry enables real-time monitoring of drug levels in biological fluids, such as blood or urine. This capability provides critical data for patient management and therapeutic efficacy, allowing healthcare professionals to make informed decisions regarding treatment.
The combination of amperometry with Flow Injection Analysis (FIA) allows for swift and effective evaluation of multiple samples, significantly decreasing processing time and enhancing throughput. Recent studies have shown that the developed FIA-BDDE method provides compared to previously published studies, highlighting ongoing innovation in this field.
To effectively apply amperometry in pharmaceutical analysis, follow these essential steps:
- Method Selection: Choose the appropriate amperometric method based on the specific analyte and matrix involved.
- Sample Preparation: Adhere to standard operating procedures to prepare samples, ensuring consistency and reliability in results.
- Measurement Execution: Conduct measurements using a calibrated setup, maintaining control over all relevant parameters to ensure accuracy.
- Data Analysis: Utilize calibration curves to interpret the data and determine the concentration of the analyte in the samples.
Square-wave polarography has emerged as a quick method that significantly reduces evaluation time while enabling efficient scans of potential ranges. This is especially advantageous for examining metal ions and inorganic anions. The effectiveness of this method is backed by case studies showcasing its rapid analysis capabilities.
Pharmaceutical professionals have noted the effectiveness of amperometry for drug quantification, highlighting its reliability and precision. As market trends indicate a growing reliance on these techniques, the integration of amperometry into pharmaceutical quality control processes is becoming increasingly essential for ensuring product safety and efficacy. As one study concluded, "The null hypothesis was supported by the paired two-tailed test, which yielded a computed t-value of 0.906, less than the critical value (2.776)," underscoring the statistical validation of these methods.
Conclusion
Amperometry is a cornerstone technique in pharmaceutical analysis, providing unmatched accuracy in quantifying active pharmaceutical ingredients and ensuring the integrity of drug formulations. By examining the fundamental components of amperometric systems—working, reference, and counter electrodes—professionals can deepen their understanding of the method's operational nuances, which directly affect measurement reliability. The versatility of amperometric techniques, including various methods designed for specific analytical challenges, highlights their critical role in both current and future pharmaceutical applications.
Equipping laboratories with high-quality electrochemical workstations and properly calibrated electrodes is essential for achieving precise results. The importance of rigorous calibration processes cannot be overstated, as they are vital for minimizing errors and maintaining measurement accuracy over time. This meticulous approach to calibration not only reinforces the reliability of results but also supports the broader goals of quality control and method validation in pharmaceutical settings.
The applications of amperometry extend beyond simple quantification; they include quality control, real-time monitoring, and innovative techniques such as flow injection analysis. As the demand for rapid and reliable analytical methods increases, the integration of amperometric techniques into pharmaceutical analysis becomes indispensable. Embracing these advancements enhances analytical capabilities and ensures compliance with regulatory standards, ultimately safeguarding patient health and product safety. As the pharmaceutical landscape evolves, mastering amperometric techniques will remain a priority for professionals committed to excellence in drug development and quality assurance.




