Overview
This article delves into the effective synthesis of copolymers utilizing divinylbenzene styrene and styrene, highlighting the critical importance of comprehending their properties and adhering to systematic preparation and polymerization techniques. By detailing the chemical characteristics of both components, it provides a comprehensive step-by-step guide for synthesis. Furthermore, it addresses common challenges that may arise during the process, ensuring the production of high-quality copolymers tailored for specific applications. Understanding these elements is essential for achieving optimal results in copolymer synthesis.
Introduction
Mastering the synthesis of copolymers using divinylbenzene and styrene stands as a pivotal skill in the domain of polymer chemistry, where precise techniques can markedly improve material properties. This guide explores the fundamental steps and methodologies for effectively integrating these two essential components, shedding light on their distinct characteristics and the advantages they confer to the final product.
Nevertheless, the path to successful copolymerization is laden with challenges—how can one guarantee optimal outcomes while adeptly navigating the common pitfalls inherent in the synthesis process?
Understand Divinylbenzene and Styrene Properties
To effectively synthesize copolymers using divinylbenzene styrene and other components, understanding their chemical properties is essential.
Divinylbenzene styrene serves as a cross-linking agent that significantly enhances the structural integrity of polymer blends. Its two vinyl groups facilitate polymerization, resulting in a robust three-dimensional network. Key properties of divinylbenzene styrene include high reactivity, stemming from its vinyl groups, which makes divinylbenzene styrene an ideal candidate for copolymerization processes. Additionally, DVB provides essential thermal stability to the polymer blend, allowing it to endure high temperatures. This characteristic is crucial for applications demanding heat resistance. Notably, thermal analysis has shown that synthesized DVB-TEVS materials are thermally stable up to at least 330 °C, confirming their suitability for high-temperature applications.
Styrene, a , adds flexibility and processability to composite materials. Its hydrophobic nature influences the solubility and compatibility of the copolymer in various solvents, thereby affecting its performance in practical applications. Furthermore, with a glass transition temperature (Tg) around 100°C, styrene plays a critical role in determining the thermal properties of the final product, influencing its usability across different temperature ranges.
By thoroughly grasping these characteristics, one can predict the behavior of the polymer blend under varying conditions, which is essential for enhancing the synthesis process to fulfill specific application needs. As highlighted by specialists, 'The thermal assessment demonstrated that the synthesized materials with divinylbenzene styrene were thermally stable up to at least 330 °C,' emphasizing the significance of thermal stability in polymers.
Prepare Copolymers Using Styrene and Divinylbenzene
To prepare copolymers using styrene and divinylbenzene, follow these essential steps:
-
Select the Ratios: Begin by determining the desired properties of your copolymer through appropriate ratios of divinylbenzene to styrene. A widely accepted starting point is a 70:30 ratio, although adjustments may be necessary based on specific application requirements.
-
Gather Materials: Ensure that you have all necessary materials on hand:
- Styrene monomer
- Divinylbenzene monomer
- Initiator (e.g., benzoyl peroxide)
- Solvent (if necessary, such as toluene)
- A reaction vessel equipped for stirring
-
Set up the reaction by combining the styrene and divinylbenzene within the reaction vessel in a fume hood. If you are using a solvent, incorporate it into the mixture to facilitate polymer formation.
-
Add Starter: Introduce the starter to the mixture to initiate the polymerization process. The quantity of catalyst can vary; however, a typical range is between 1-5% of the total monomer weight.
-
Control Temperature: Maintain the reaction temperature according to the initiator's specifications, generally between 60-80°C, to ensure optimal rates of chemical change.
-
Stir the Mixture: Continuously stir the mixture to guarantee a uniform distribution of the reactants, which promotes effective polymerization.
-
Monitor the Reaction: Vigilantly observe the reaction's progress by sampling at intervals to assess viscosity changes or other indicators of polymer formation.
By adhering to these steps, you can successfully prepare a copolymer tailored to meet your specific application needs.
Execute Polymerization Techniques for Optimal Results
To execute polymerization techniques effectively, it is essential to follow these steps:
- Initiate Polymerization: Begin by incorporating the initiator and allowing the process to continue for a predetermined time, typically between 2 to 6 hours, depending on the desired molecular weight.
- Control Reaction Conditions: Maintaining consistent temperature and stirring throughout the reaction is crucial. Fluctuations can lead to inconsistent polymer formation, adversely impacting the final product's properties. Researchers emphasize that controlling these conditions is vital for optimal results in polymer synthesis. Notably, the activation energy for synthesis is approximately 40 kcal/mol, underscoring the necessity for precise control during the process.
- Stop the Reaction: After the required reaction time, cool the mixture or introduce a terminating agent, such as hydroquinone, to effectively halt the process.
- Purify the Copolymer: It is imperative to remove unreacted monomers and solvents through precipitation in a non-solvent (e.g., methanol) or via filtration. This purification step is critical for ensuring the integrity and performance of the final product.
- Characterize the Copolymer: Employ techniques such as gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) spectroscopy to analyze the copolymer. These methods confirm its , providing insights into its suitability for specific applications. Significantly, GPC-NMR can be utilized to observe polymer formation processes and verify monomer sequences, enhancing comprehension of the polymer's characteristics.
- Optimize Conditions: Based on characterization results, refine conditions for subsequent batches to consistently achieve desired properties. Real-world examples demonstrate that adjusting factors such as initiator concentration and temperature can significantly enhance polymer performance. For instance, research has shown that the output of blended polymers increased from approximately 52% to 75% with elevated divinylbenzene styrene levels, highlighting the effect of reaction conditions on production outcomes.
By diligently applying these synthesis methods, you can ensure that the produced blend meets necessary criteria for your applications, paving the way for innovative advancements in material science.
Troubleshoot Common Polymer Synthesis Issues
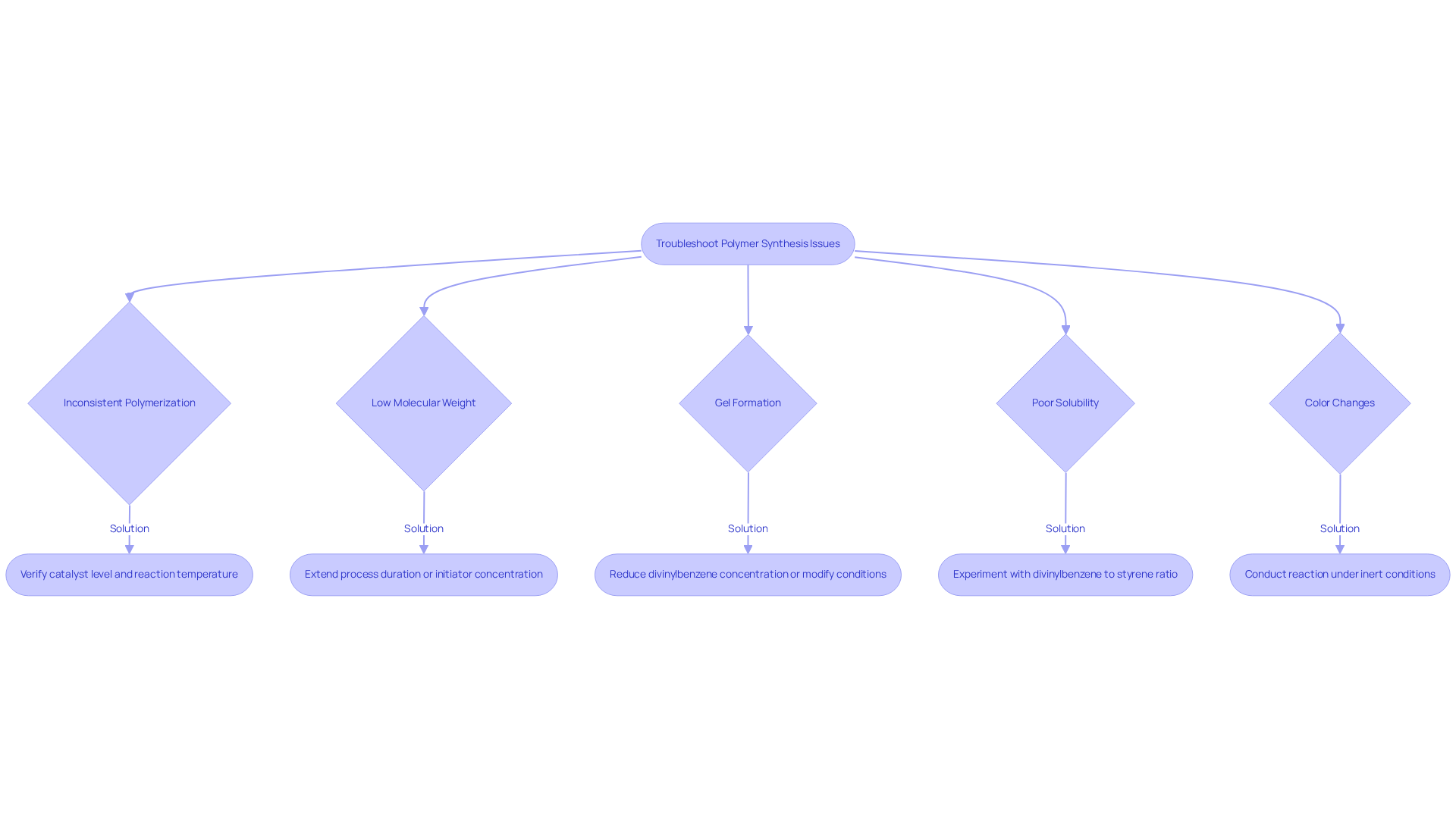
When synthesizing copolymers, various issues may arise that require careful attention. Understanding these is crucial for successful outcomes.
- Inconsistent Polymerization: This issue often stems from the catalyst level. To ensure optimal results, verify that the starting agent's concentration is appropriate and that the reaction temperature remains stable. Adjusting the quantity of the starter or the temperature can significantly enhance consistency. As Grzegorz Przesławski notes, "The rise in the initiating system concentration leads to a higher number of generated radicals and an accelerated rate of polymer formation, which reduces the setting time."
- Low Molecular Weight: If the resulting copolymer exhibits low molecular weight, consider extending either the process duration or the initiator concentration. Such adjustments can facilitate further polymerization, thereby enhancing the molecular weight of the product.
- Excessively high concentrations of divinylbenzene styrene can lead to gel formation. To mitigate this issue, reduce the amount of divinylbenzene or modify the reaction conditions to prevent excessive cross-linking, which can result in gelation.
- Poor solubility in the polymer blend may arise from an inappropriate ratio of divinylbenzene styrene to styrene. Experimenting with various ratios can improve solubility in the desired solvent, ensuring better performance in applications.
- Color Changes: Unwanted color alterations in the blend often indicate degradation. Conducting the reaction under inert conditions is vital to prevent oxidation and degradation of the monomers, thus maintaining the integrity of the final product.
By being aware of these common issues and their solutions, you can troubleshoot effectively and enhance the success rate of your copolymer synthesis.
Conclusion
Mastering the synthesis of copolymers using divinylbenzene styrene is an essential skill for innovators in materials science. By grasping the unique properties of divinylbenzene and styrene, one can effectively tailor copolymers to meet specific application demands. The ability to manipulate ratios, control reaction conditions, and troubleshoot common synthesis issues is crucial for achieving optimal results in polymerization processes.
This guide outlines the essential steps for preparing copolymers, from selecting appropriate ratios and gathering necessary materials to executing polymerization techniques and addressing potential challenges. Key insights into the properties of divinylbenzene and styrene underscore their roles in enhancing thermal stability, flexibility, and overall performance of the copolymer, ensuring a robust end product suitable for various applications.
In conclusion, successful copolymer synthesis transcends mere procedural adherence; it requires a deep understanding of the underlying chemistry and the ability to adapt techniques to overcome challenges. As the field of polymer science evolves, staying informed about best practices and emerging trends will empower researchers and practitioners to push the boundaries of what is possible with divinylbenzene styrene copolymers. Embracing this knowledge can lead to innovative advancements and practical applications that significantly impact industries reliant on high-performance materials.




