Overview
This article presents a comprehensive step-by-step guide on effectively utilizing an HPLC method transfer calculator. It underscores the significance of adapting HPLC techniques to new conditions, all while ensuring efficiency and accuracy. Detailed instructions are provided for identifying key parameters and troubleshooting common issues, alongside leveraging additional resources. This demonstrates how these calculators not only streamline transitions but also enhance laboratory workflows, ultimately reinforcing the critical role they play in modern scientific practices.
Introduction
The world of high-performance liquid chromatography (HPLC) is rapidly evolving, making method transfers a critical component of laboratory efficiency. HPLC method transfer calculators serve as invaluable tools that streamline the adaptation of existing methods to new conditions, significantly minimizing waste and enhancing reproducibility. Yet, the process of transferring methods can present numerous challenges, prompting many professionals to ask: how can one ensure a seamless transition while preserving the integrity of the original technique?
This guide explores the step-by-step utilization of HPLC method transfer calculators, providing insights into best practices and addressing common issues, ultimately empowering laboratory technicians and chemists in their analytical endeavors.
Understand HPLC Method Transfer Calculators
HPLC transfer tools serve as essential resources for chemists and laboratory technicians, facilitating the adaptation of current HPLC practices to new conditions, such as transitioning to UHPLC systems. These devices consider critical factors, including column sizes, flow rates, and solvent mixtures, ensuring that techniques remain efficient and consistent. By utilizing these tools, laboratories can significantly enhance efficiency, reducing the reliance on trial-and-error methods and minimizing solvent waste.
For instance, when transitioning from a 5 μm to a 3 μm particle size, nearly equivalent separations can be achieved at the same reduced velocity, demonstrating the tools' ability to maintain procedural integrity. However, it is imperative to understand that more precise scaling necessitates actual measured values of column volumes, which are influenced by factors such as particle size and structure. Familiarity with key parameters, including gradient delay volume and injection volume, is essential for accurate calculations and effective technique adaptation.
The importance of the extends beyond mere convenience; it is pivotal in enhancing laboratory efficiency. By streamlining the procedure transition process, laboratories can utilize the HPLC method transfer calculator to conserve time and resources, ultimately resulting in more reliable analytical outcomes. Recent studies underscore that employing a tolerance interval approach for procedure transition provides a scientifically valid basis for ensuring equivalency between laboratories. Davy Guillarme emphasizes that refining this approach effectively can save analysts both time and resources, further highlighting the tools' significance in modern analytical practices. Additionally, these devices can evaluate solvent usage reduction during procedure transitions, showcasing their role in promoting sustainability within laboratory operations.
Follow Step-by-Step Instructions for Using the Calculator
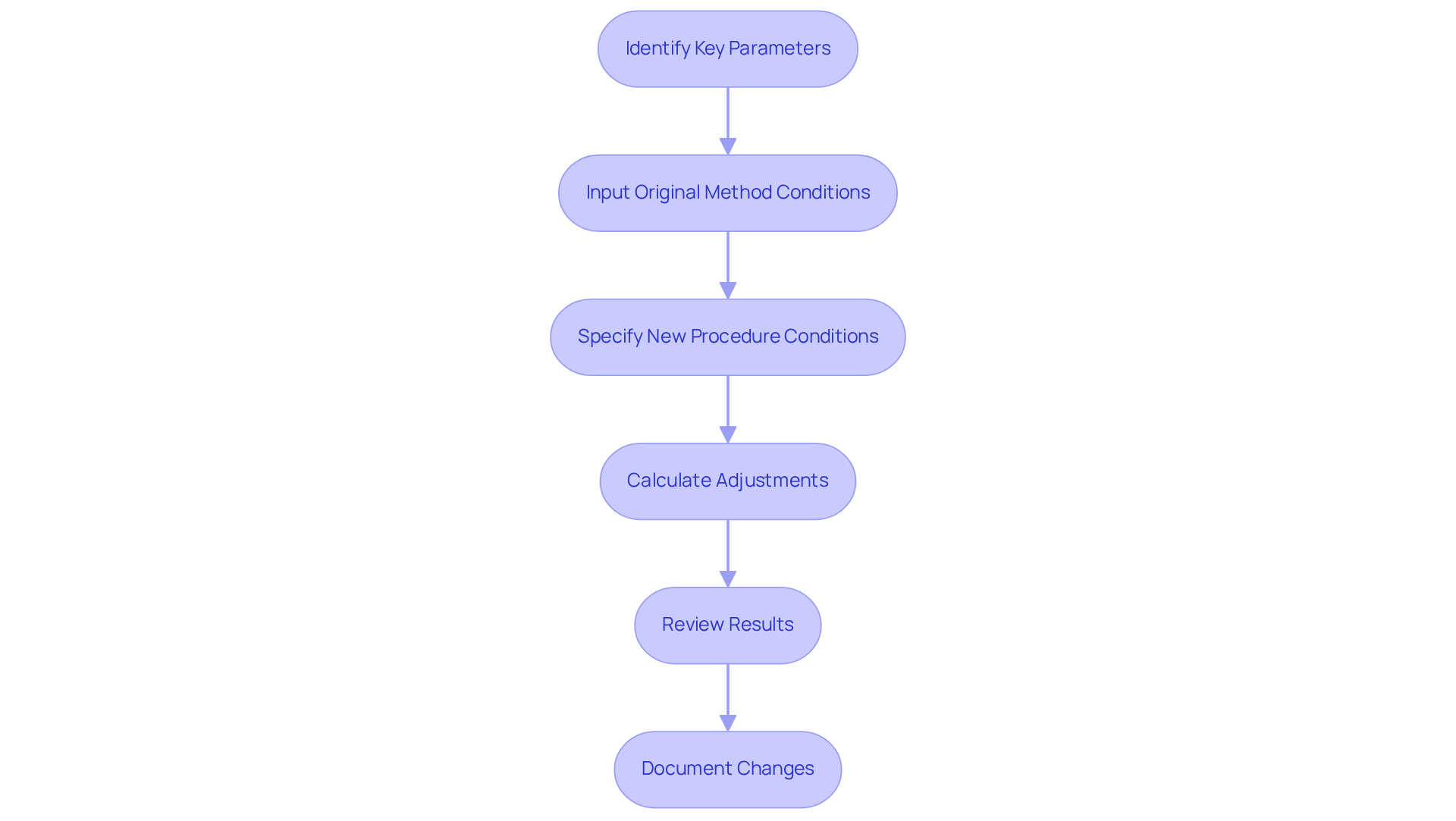
To effectively utilize an HPLC method transfer calculator, follow these essential steps:
- Identify Key Parameters: Begin by gathering the initial conditions of your HPLC procedure, including original column dimensions (length, diameter, and particle size), flow rate, and solvent composition.
- Input Original Method Conditions: Enter the identified parameters into the device. Most devices feature sections for each variable, enabling precise representation of your current approach.
- Specify New Procedure Conditions: Next, input the desired conditions for the new procedure, which may involve changes in column dimensions or flow rates.
- Calculate Adjustments: After entering all parameters, utilize the device to generate the new procedure conditions. This typically includes adjusted flow rates, injection volumes, and gradient times.
- Review Results: Carefully examine the output provided by the calculator. Ensure that the new conditions align with your experimental goals and maintain the integrity of the original approach. For instance, case studies have demonstrated that systematic parameter adjustments can enhance technique performance, such as the automated kinetic profiling in catalytic systems, which resulted in a five-fold increase in reaction rates.
- Document Changes: Finally, record the alterations made to the procedure for future reference and adherence to laboratory protocols. This step is crucial for maintaining a clear record of technique adaptations.
In practice, implementing these steps with the HPLC method transfer calculator can lead to significant time savings. Utilizing simplifies the modification process, enabling quicker transitions between techniques while ensuring precision. As highlighted by Meiyu Shen from the US FDA, establishing suitable values for procedure transfer is vital, as it ensures that the process remains appropriate for its intended application post-transfer. By adhering to these guidelines and integrating insights from case studies and expert opinions, pharmaceutical lab managers can effectively optimize their laboratory workflows.
Troubleshoot Common Issues During Method Transfer
Transferring techniques can present several common challenges that require effective troubleshooting when using an HPLC method transfer calculator. Here are some essential tips to navigate these issues:
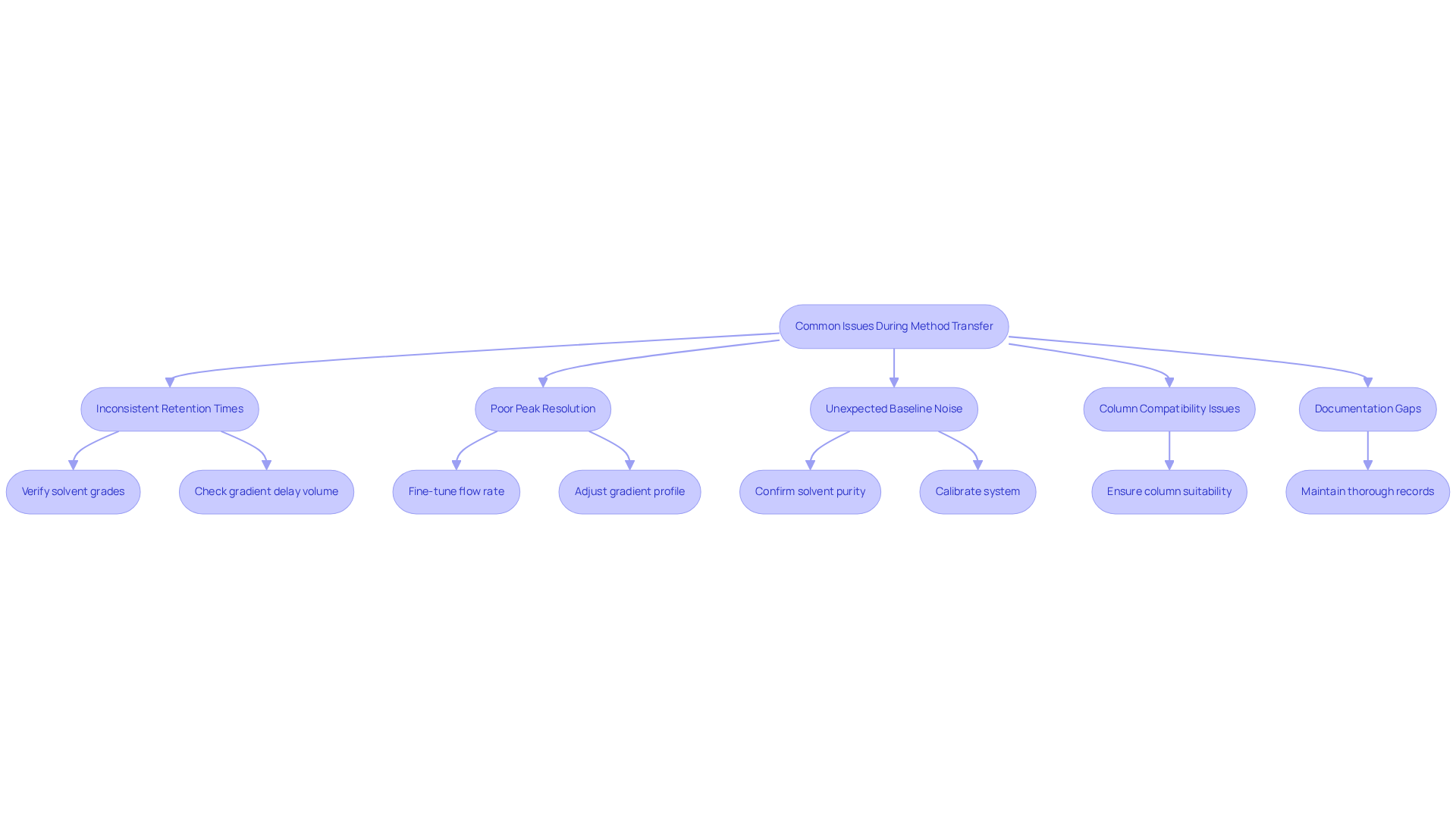
- Inconsistent Retention Times: Significant differences in retention times between the original and new approaches often stem from variations in gradient delay volume. It is crucial to verify that the same grades of solvents are employed, as using low-grade additives can lead to unforeseen 'ghost peaks' in gradient elution techniques. Notably, many laboratories report retention time inconsistencies due to overlooked parameters, underscoring the need for meticulous attention to detail.
- Poor Peak Resolution: If peak resolution is compromised, consider fine-tuning the flow rate or gradient profile. Even minor adjustments can dramatically enhance separation quality. Many laboratories have observed that retention time inconsistencies often arise from overlooked parameters, reinforcing the importance of careful calibration.
- Unexpected Baseline Noise: may arise from differences in instrument sensitivity or solvent purity. To mitigate this, confirm that all solvents are of high quality and that the system is calibrated correctly. Regular performance qualification tests can help maintain system integrity and ensure reliable results.
- Column Compatibility Issues: Ensure that the new column is suitable for the technique being transferred. Differences in particle size or column chemistry can lead to unexpected chromatographic outcomes, highlighting the significance of comprehensive procedure documentation.
- Documentation Gaps: Maintaining thorough records of both the original and altered processes is essential for compliance and efficient troubleshooting during process validation. This practice not only aids in identifying issues but also supports a smoother transition between systems, thereby enhancing overall operational efficiency with the HPLC method transfer calculator.
Additional Resources for HPLC Method Transfer
To enhance your HPLC method transfer process, consider leveraging the following resources:
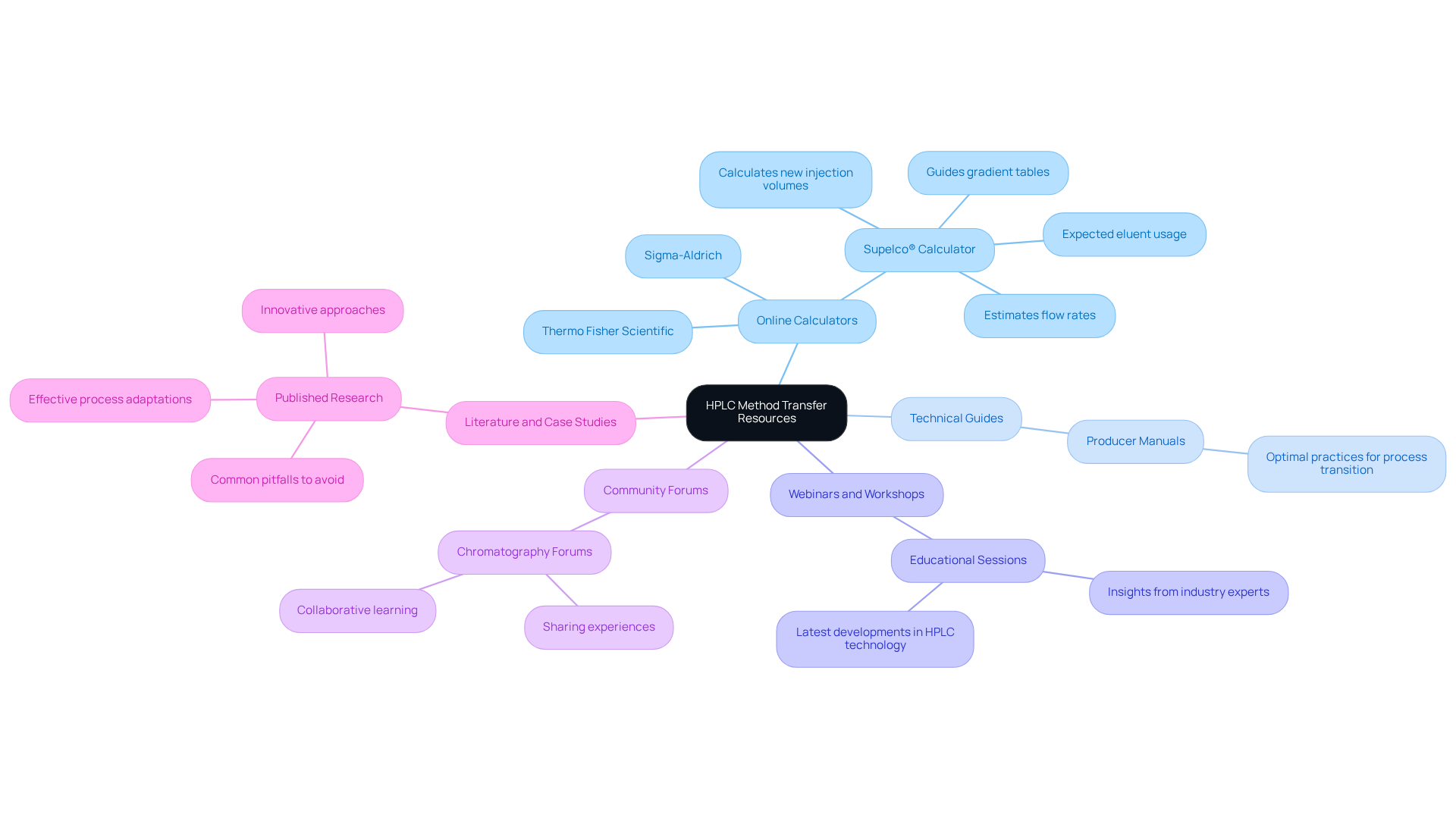
- Online Calculators: Platforms such as Thermo Fisher Scientific and Sigma-Aldrich provide intuitive HPLC method transfer calculators. The HPLC method transfer calculator from Supelco® can calculate new injection volumes, flow rates, gradient tables, and expected eluent usage based on user-defined parameters. This functionality makes transitions smoother and more efficient. However, it is crucial to note that precise scaling of techniques requires actual measured values of column volumes, which depend on factors such as particle size and column packing.
- Technical Guides: Numerous producers offer extensive manuals outlining optimal practices for process transition. These resources are essential for grasping the intricacies of various systems and ensuring accurate results.
- Webinars and Workshops: Engaging in educational webinars or workshops can provide practical experience and insights from industry experts. These sessions frequently address the latest developments in HPLC technology and process adaptation techniques.
- Community Forums: Participating in online chromatography forums allows you to connect with fellow professionals who have encountered similar challenges. Sharing experiences and solutions can lead to valuable insights and support, thereby enhancing collaborative learning within the chromatography community.
- Literature and Case Studies: Examining published research on effective process adaptations can provide practical examples and strategies relevant to your laboratory. These case studies often highlight innovative approaches and common pitfalls to avoid, thereby enhancing your understanding of effective method transfer.
Conclusion
Mastering the HPLC method transfer calculator is essential for successful transitions between different chromatography systems. By effectively utilizing these calculators, chemists and laboratory technicians can ensure that their methodologies remain consistent and efficient, ultimately enhancing the reliability of their analytical outcomes.
Understanding key parameters, following systematic steps, and troubleshooting common issues when using these calculators are critical. Identifying original method conditions and documenting changes are vital for maintaining procedural integrity. Additionally, leveraging resources such as online calculators, technical guides, and community forums can provide invaluable support throughout the method transfer process.
Embracing the HPLC method transfer calculator streamlines laboratory workflows and promotes sustainability by minimizing solvent waste. As laboratories continue to evolve, adopting these best practices is essential for maintaining high standards in analytical chemistry. Engaging with the latest tools and resources empowers professionals to navigate the complexities of method transfer, ensuring they remain at the forefront of their field.




