Overview
The article emphasizes the principles of column chromatography and its vital role in achieving laboratory success. Mastering key concepts, such as stationary and mobile phases, alongside mechanisms like polarity and size exclusion, is crucial for effectively isolating components across various fields, including pharmaceuticals and environmental analysis. This mastery ensures high-quality outcomes in laboratory settings, reinforcing the necessity of understanding these principles for any professional aiming for excellence in scientific practices.
Introduction
Column chromatography stands as a cornerstone technique in analytical chemistry, recognized for its unparalleled ability to separate complex mixtures into individual components. By mastering the principles of this method, laboratory professionals can significantly enhance their efficiency and accuracy across a range of applications, from pharmaceuticals to environmental monitoring.
Yet, what are the critical mechanisms that govern this separation process? How can one effectively implement the procedure to achieve optimal results? Exploring these questions not only uncovers the intricacies of column chromatography but also highlights its profound impact on scientific research and public health.
Define Column Chromatography: Key Concepts and Terminology
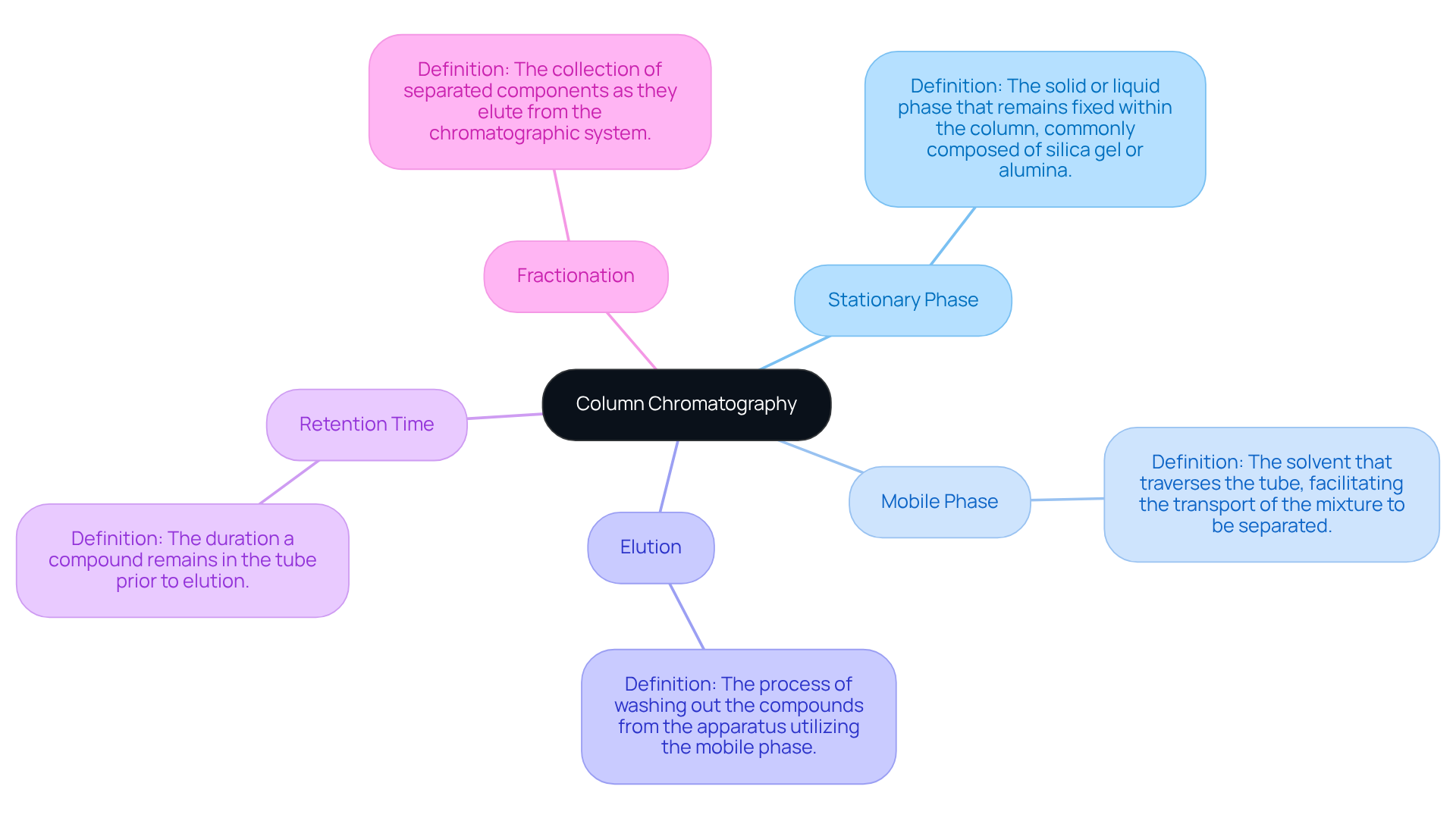
The column chromatography principle serves as a vital separation technique for isolating individual components from a mixture. Understanding key terms is essential for in laboratory environments.
- The Stationary Phase refers to the solid or liquid phase that remains fixed within the column, commonly composed of silica gel or alumina.
- The Mobile Phase is the solvent that traverses the tube, facilitating the transport of the mixture to be separated.
- Elution describes the process of washing out the compounds from the apparatus utilizing the mobile phase.
- Retention Time indicates the duration a compound remains in the tube prior to elution, while
- Fractionation involves the collection of separated components as they elute from the chromatographic system.
Mastering these concepts is crucial for proficiently communicating and employing the column chromatography principle in laboratory settings.
Explain the Principles of Column Chromatography: Mechanisms of Separation
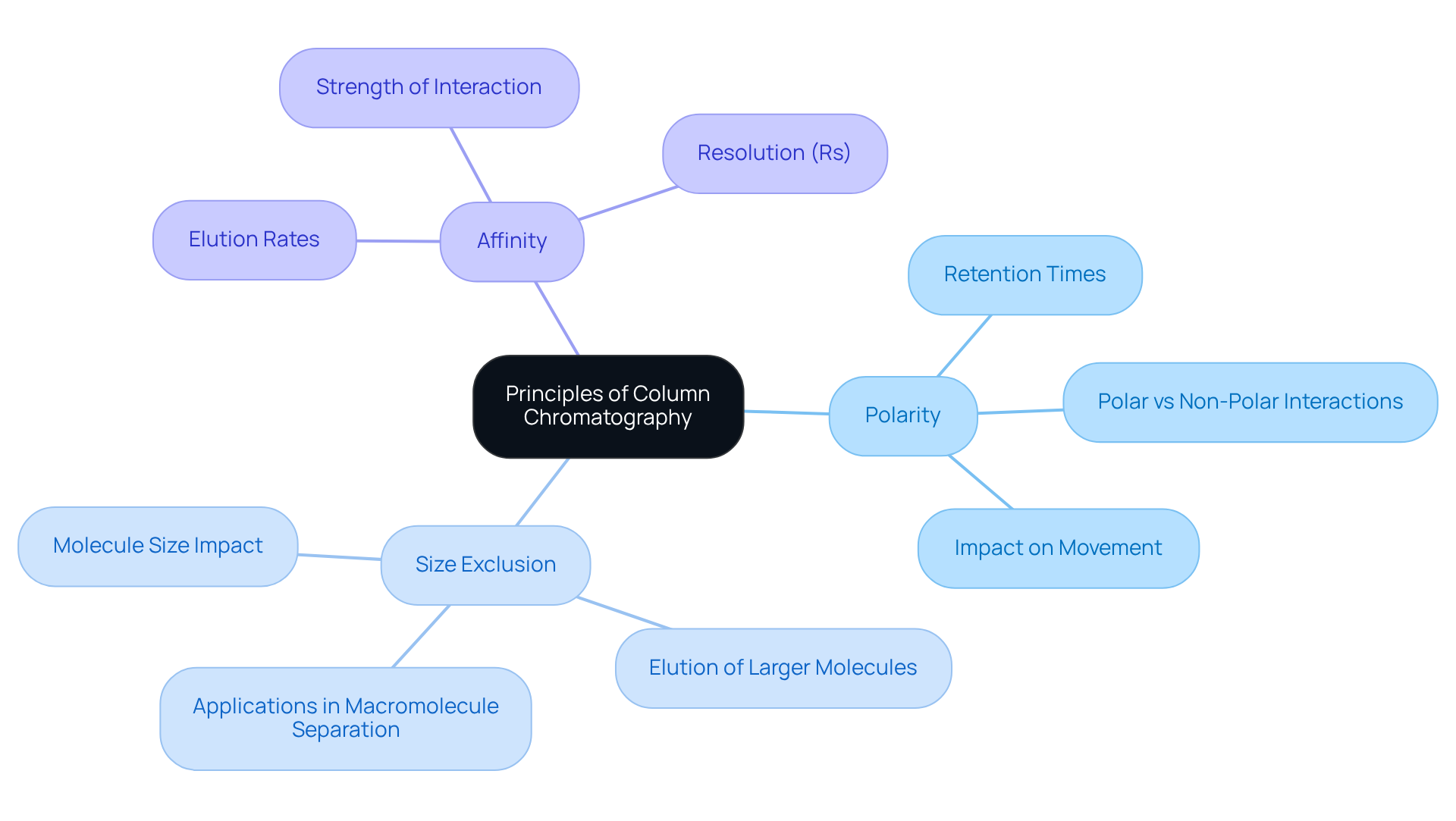
Column separation is based on the column chromatography principle, which involves differential adsorption and partitioning between the stationary and moving states. The separation occurs due to several key mechanisms:
- Polarity: Compounds with differing polarities interact variably with the stationary phase, resulting in distinct retention times. Polar substances exhibit a stronger attraction to polar stationary materials, causing them to traverse the separation system at a slower pace. As noted by Till from the University of Cambridge, 'The polarity of substances greatly affects their movement and division during the process of chromatographic analysis.' This highlights the critical role polarity plays in separation efficiency.
- Size Exclusion: Larger molecules may be hindered from entering the pores of the stationary medium, which directly impacts their elution. This size exclusion effect is particularly vital in applications requiring the separation of macromolecules, as emphasized in recent studies on size exclusion chromatography. Understanding this mechanism is essential for involving larger compounds.
- Affinity: The strength of interaction between the compounds and the stationary medium significantly influences their elution rates. Compounds exhibiting a greater affinity for the stationary phase will elute more slowly, while those with lesser affinity will move through the tube more rapidly. The resolution (Rs) of two peaks is considered baseline resolved if Rs ≥ 1.5, indicating effective distinction between the components.
These mechanisms facilitate the efficient division of complex mixtures according to their chemical characteristics, thereby establishing the column chromatography principle as a versatile instrument in analytical chemistry. Recent studies underscore the importance of comprehending chromatographic efficiency, selectivity, and retention in optimizing separation outcomes, thereby reinforcing the necessity for high-quality scientific instruments in laboratory environments.
Detail the Procedure for Performing Column Chromatography: Step-by-Step Guide
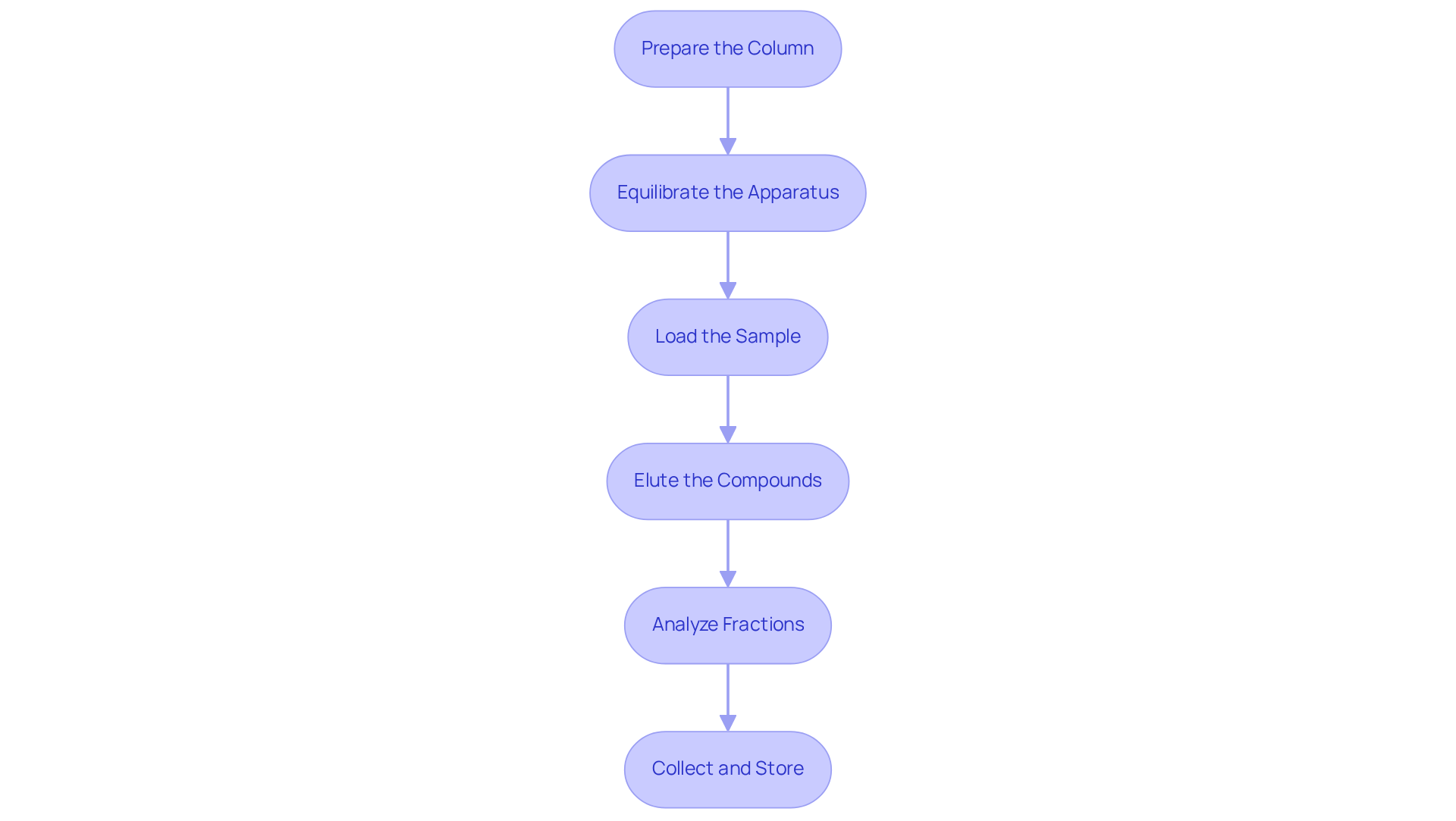
To perform column chromatography effectively, it is essential to follow these steps meticulously:
- Prepare the Column: Begin by selecting a column size that corresponds to your sample volume. For optimal packing, employ the slurry method using silica gel as the stationary medium. Mix it with a non-polar solvent, such as 15 mL of hexanes, to eliminate air bubbles and ensure a uniform bed. The mass of the sample should generally not exceed 1-5% of the stationary material mass.
- Equilibrate the Apparatus: Introduce the mobile substance into the apparatus until complete saturation is achieved. This step is crucial for establishing a , which is vital for reproducible results.
- Load the Sample: Carefully apply the sample mixture to the top of the vertical structure. It is important to avoid disturbing the stationary layer during this process to maintain the integrity of the packing.
- Elute the Compounds: Gradually add the mobile phase to the column, collecting fractions at regular intervals. This controlled addition facilitates effective differentiation based on differential adsorption.
- Analyze Fractions: Utilize methods such as thin-layer analysis (TLA) to observe the division process. This allows for real-time assessment of compound elution and purity.
- Collect and Store: Once the desired compounds have been separated, collect the fractions and store them appropriately for subsequent analysis.
Implementing these best practices not only enhances the efficiency of your separation techniques based on the column chromatography principle but also minimizes common errors that can lead to poor resolution and compromised outcomes. For example, improper packing may result in uneven flow and band broadening, while inadequate equilibration can adversely affect the reproducibility of your separations. As an expert aptly stated, "There’s nothing like having the practice of doing it yourself to know how to tackle issues and troubleshoot a procedure." By adhering to this organized method, you can achieve high-quality outcomes in your separation techniques.
Explore Applications of Column Chromatography: From Pharmaceuticals to Environmental Analysis
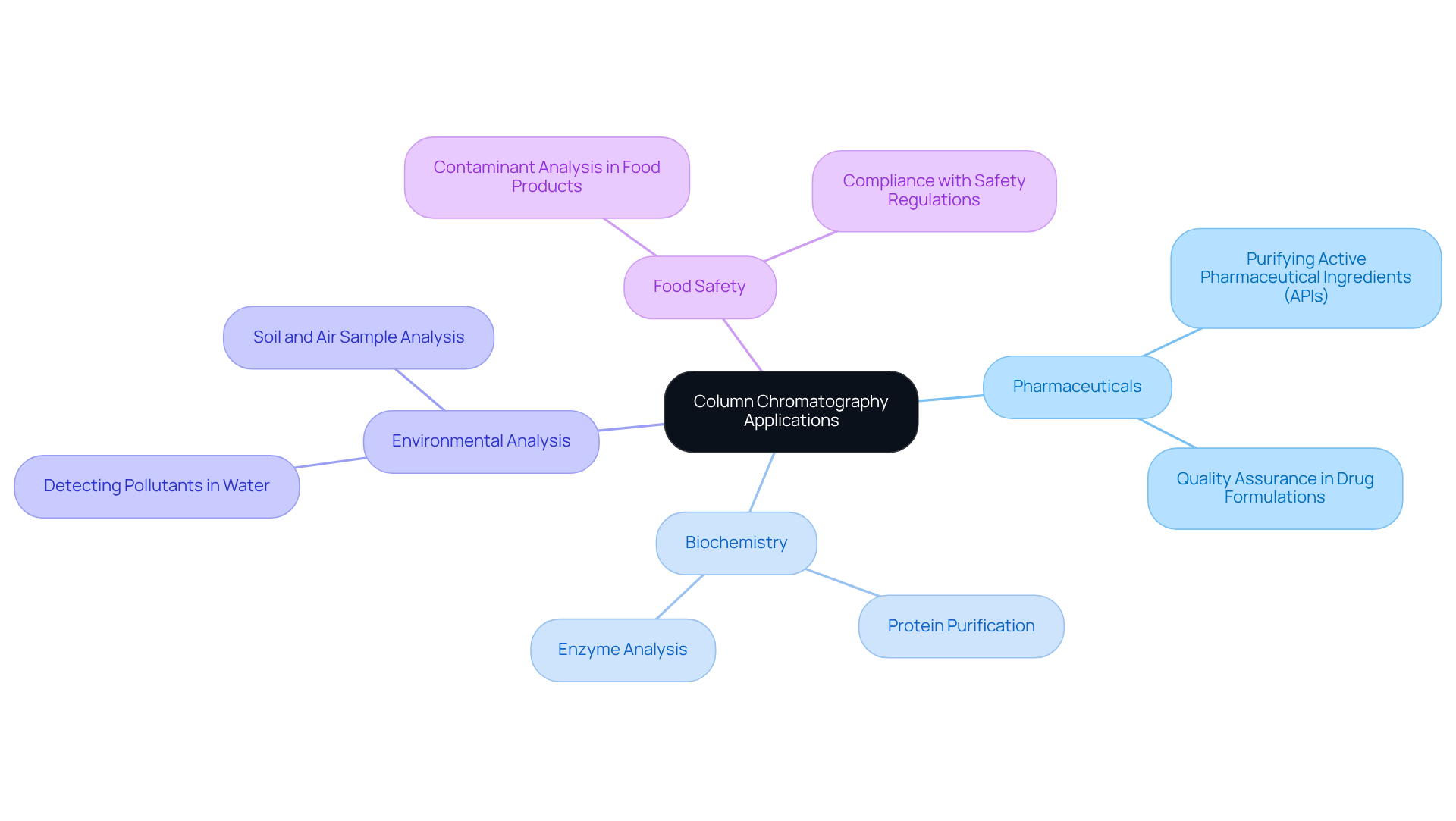
The column chromatography principle plays a crucial role in various fields, demonstrating its importance and versatility. In the pharmaceutical industry, the column chromatography principle is essential for purifying active pharmaceutical ingredients (APIs) and ensuring the quality of drug formulations. This process not only enhances the efficacy of medications but also by maintaining rigorous quality standards.
In the realm of biochemistry, the column chromatography principle facilitates protein purification and enzyme analysis, allowing researchers to isolate specific proteins for detailed study. This capability is vital for advancing our understanding of biological processes and developing novel therapeutic strategies.
Environmental analysis also benefits significantly from this technique, as it is employed to detect and quantify pollutants in water, soil, and air samples. Such applications contribute to comprehensive environmental monitoring efforts, ensuring the safety and health of ecosystems and communities alike.
Furthermore, in the context of food safety, the column chromatography principle is utilized for analyzing food products for contaminants, thereby ensuring compliance with safety regulations. This practice is critical in protecting public health and maintaining consumer trust in food products.
These diverse applications underscore the indispensable role of column chromatography in advancing scientific research and ensuring public safety, highlighting the need for high-quality scientific instruments in laboratory settings.
Conclusion
Mastering the principles of column chromatography is essential for achieving success in laboratory environments. This technique serves as a powerful method for separating and purifying compounds and plays a critical role in various scientific fields, from pharmaceuticals to environmental analysis. Understanding the foundational concepts, mechanisms of separation, and the procedural steps involved is key to harnessing the full potential of this versatile analytical tool.
Throughout this article, we explored key aspects of column chromatography, including:
- The importance of the stationary and mobile phases
- Mechanisms of separation such as polarity, size exclusion, and affinity
- The meticulous step-by-step procedures necessary for effective execution
Each of these components contributes to optimizing separation outcomes, ensuring high-quality results in laboratory applications. Furthermore, the diverse applications of column chromatography highlight its significance in improving drug formulations, conducting protein studies, monitoring environmental pollutants, and ensuring food safety.
In light of the critical insights provided, it is clear that mastering column chromatography is not just about understanding its mechanics but also about recognizing its profound impact on scientific research and public health. As the field continues to evolve with advancements in techniques and applications, staying informed and skilled in column chromatography practices will remain vital for laboratory professionals. Embracing these principles can lead to enhanced research outcomes and contribute to the broader goals of scientific innovation and safety.




