Overview
The primary focus of this article centers on the selection of the appropriate HPLC stationary phase to ensure effective analysis in High-Performance Liquid Chromatography (HPLC). It is essential to carefully consider various factors, including:
- The nature of the analyte
- Separation goals
- Mobile phase compatibility
- Cost
These elements are crucial for optimizing analytical outcomes, as they directly influence the efficiency and resolution of the separation process. By understanding these key considerations, laboratory professionals can significantly enhance their analytical capabilities and achieve superior results.
Introduction
In the realm of analytical chemistry, High-Performance Liquid Chromatography (HPLC) emerges as a pivotal technique for the separation and analysis of complex mixtures. As industries increasingly rely on precise analytical methods, it becomes essential for laboratory managers and scientists to understand the intricacies of HPLC.
Each decision, from the selection of the appropriate stationary phase to the optimization of system components, can significantly influence the outcomes of analytical processes. With the HPLC market experiencing growth driven by technological advancements and rising demand across sectors such as pharmaceuticals, staying informed about the latest trends and methodologies is crucial.
This article explores the fundamental aspects of HPLC, critical factors in stationary phase selection, and the diverse types of stationary phases available, equipping professionals with the knowledge necessary to enhance their analytical capabilities.
Understand High-Performance Liquid Chromatography (HPLC)
(HPLC) stands as a sophisticated analytical method, essential for the division, identification, and quantification of components within complex mixtures. This technique necessitates the flow of a liquid sample through a column filled with a fixed medium, complemented by a moving component that facilitates the transport of the sample through the column. The effectiveness of separation hinges primarily on the interactions between the sample components and the stationary medium, making the selection of the appropriate stationary medium critical for achieving high-quality outcomes.
Key components of HPLC systems encompass the pump, injector, column, and detector, each playing a vital role in the system's overall performance. For instance, the pump must ensure a steady flow rate to guarantee reproducibility, while the injector must accurately introduce the sample into the moving medium. The column, packed with the stationary phase, is where the actual separation transpires, and the detector measures the components as they elute.
In 2024, the high-performance liquid chromatography market was valued at approximately USD 4.99 billion, with ongoing advancements in technology poised to drive further growth and competition in 2025. Factors such as swift drug manufacturing and stringent regulatory standards are propelling the demand for high-performance liquid chromatography systems across various industries, including pharmaceuticals and medical diagnostics. JM Science Inc. offers a comprehensive selection of high-quality liquid chromatography columns and accessories, featuring Shodex, CapcellPak, and Reprosil columns, all available at remarkably favorable prices, which are essential for enhancing analytical processes. This growth underscores the importance of remaining abreast of the latest advancements in chromatography technology.
Understanding the components of high-performance liquid chromatography is not merely advantageous but crucial for laboratory managers aiming to enhance their analytical processes. As emphasized by industry specialists, "The accessories segment constitutes a vital part of the high-performance liquid chromatography market, offering essential support elements that improve the functionality and efficiency of these systems." This highlights the importance of selecting , which can significantly influence separation efficiency and analysis outcomes.
Moreover, case studies reveal that the high costs associated with high-performance liquid chromatography devices and supplies present challenges for small and medium-sized enterprises, as these expenses can hinder their ability to adopt advanced analytical methods. Addressing these cost-related challenges is vital for the widespread acceptance of high-performance liquid chromatography across various end-user segments. By staying informed about the latest advancements and understanding the essential elements of high-performance liquid chromatography, laboratory managers can enhance their analytical capabilities and ensure compliance with evolving industry standards. Additionally, JM Science Inc. provides a variety of titrators and Karl Fischer reagents, further supporting laboratories in their analytical needs.
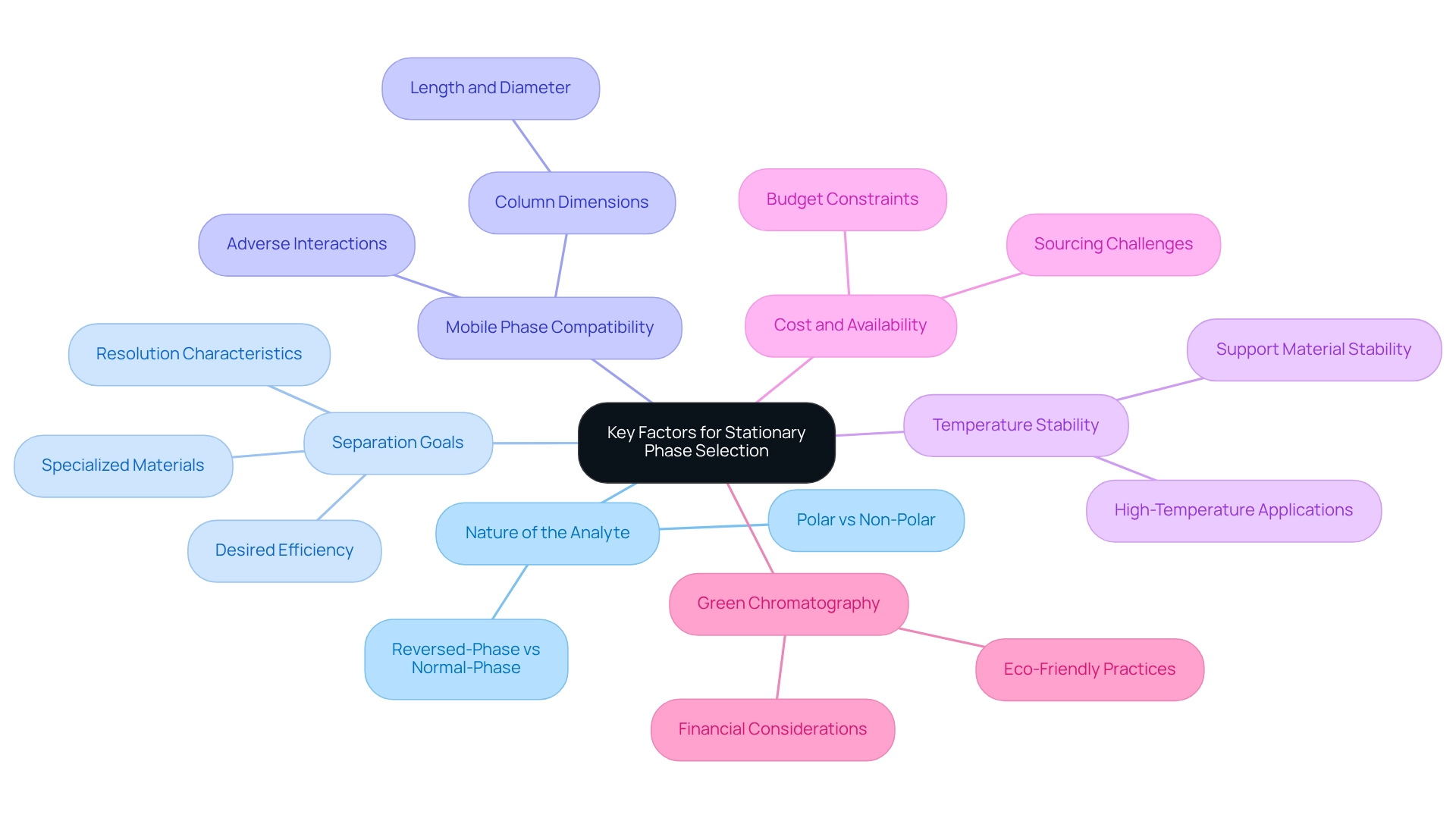
Identify Key Factors for Stationary Phase Selection
Selecting for High-Performance Liquid Chromatography (HPLC) is crucial for achieving optimal analytical outcomes. Several key factors warrant careful consideration:
- Nature of the Analyte: It is essential to determine whether the analyte is polar or non-polar, as this decision influences the choice between normal-phase and reversed-phase chromatography. For example, polar analytes typically necessitate the use of reversed-phase columns, while non-polar analytes are often better suited for normal-phase columns.
- Separation Goals: Clearly defining the desired separation efficiency and resolution is vital. The selectivity and retention characteristics of different solid supports, such as the HPLC stationary phase, significantly impact analytical results. The application of specialized materials can enhance resolution, particularly for complex mixtures.
- Mobile Phase Compatibility: Ensuring compatibility between the fixed medium and the chosen mobile phase is critical to prevent adverse interactions that could compromise process quality. It is important to confirm that the mobile component does not detrimentally affect the performance of the HPLC stationary phase. The dimensions of the column, including its length and diameter, play a pivotal role in both time and efficiency. Longer columns can enhance resolution, although they may extend analysis time, while shorter columns can accelerate the process at the potential cost of resolution.
- Temperature Stability: Assessing the thermal stability of the stationary phase is particularly important for high-temperature applications. Certain support materials are designed to withstand elevated temperatures, which can improve separation efficiency and reduce analysis duration.
- Cost and Availability: The cost and availability of the HPLC stationary phase must also be considered due to budget constraints and accessibility. While specialized phases may yield better performance, they often come at a higher cost and may be more challenging to source, impacting overall project feasibility.
- Green Chromatography: Furthermore, green chromatography aims to reduce the environmental impact of chromatographic methods by adopting sustainable practices, which can also influence financial considerations.
By meticulously evaluating these factors, laboratory supervisors can make informed decisions regarding the appropriate HPLC stationary phase that aligns with their specific analytical needs, ultimately leading to improved outcomes in their HPLC applications. As demonstrated in the case study on nano-liquid chromatography (nano-LC), this technique provides enhanced resolution and sensitivity for analyzing small sample volumes, establishing it as a valuable asset in research. Dr. Richard A. Henry, a retired analytical chemistry professor, emphasizes that much of the information presented here was initially shared in the webinar series Liquid Chromatography Column Fundamentals, underscoring the importance of selecting the right materials for optimal analytical performance. Additionally, JM Science continually updates its product offerings, ensuring that laboratory managers remain informed about the latest advancements in HPLC stationary phase.
Explore Different Types of HPLC Stationary Phases
High-Performance Liquid Chromatography (HPLC) employs various types of HPLC stationary phase, each tailored for . Understanding these stages is crucial for enhancing separation methods in pharmaceutical applications.
HPLC emerged in the 1960s, evolving from traditional column chromatography to a high-pressure system utilizing metal columns, significantly improving separation efficiency and speed. The primary support medium, known as , typically comprises C18 or C8 bonded silica, excelling in the separation of non-polar to moderately polar analytes, thus becoming a cornerstone in pharmaceutical analysis.
Normal-Phase chromatography employs polar stationary materials like silica, effectively separating polar compounds. While less common than reversed-phase, it remains vital for particular applications.
Ion-Exchange chromatography caters to charged analytes, enabling differentiation based on ionic interactions, particularly beneficial in biochemistry for protein analysis, where precise distinction is paramount.
Size-Exclusion chromatography separates molecules by size rather than chemical properties, making it ideal for analyzing polymers and biomolecules, increasingly relevant in drug formulation.
Chiral Materials are specifically designed for distinguishing enantiomers, playing a critical role in pharmaceutical applications where the purity of chiral compounds is essential. Commonly used chiral materials (CSPs) in pharmaceutical chemistry include polysaccharide benzoate and phenylcarbamate derivatives.
Mixed-Mode Stages integrate features from various fixed systems, offering versatile strategies that enhance method development, while market trends in 2025 indicate a growing preference for the HPLC stationary phase systems, particularly reversed systems, driven by their adaptability and efficiency.
Recent advancements in fixed medium technology, particularly concerning particle size and surface chemistry, are expected to further enhance efficiency and speed of partitioning. Experts highlight the advantages of reversed-phase chromatography, notably its robustness and reliability in routine analyses.
For instance, a recent case study on the purification of recombinant proteins demonstrated that reversed-phase liquid chromatography achieved over 90% purity for Mouse IgG2a Protein, underscoring its effectiveness in stringent quality control processes. The purification method involved filtering all components through a 0.45 µm or 0.2 µm filter to eliminate particles that could interfere with separation, ensuring high purity standards.
As the field of analytical chemistry progresses, the ongoing development of high-performance liquid chromatography technologies, including advancements in the HPLC stationary phase, promises to expand the application of these fixed materials across diverse scientific domains, fostering innovation and improving analytical outcomes.
Method validation should adhere to ICH guidelines to ensure compliance and reliability in pharmaceutical applications. As emphasized by Skoog et al., a solid understanding of analytical chemistry fundamentals is essential for effective method development and application.
Select the Appropriate Stationary Phase for Your Application
Choosing the appropriate HPLC stationary phase for your HPLC application is essential for attaining optimal distinction and analysis. The selection of the HPLC stationary phase significantly affects the interaction between the analytes and the column, thus impacting the efficiency of the division and resolution. To ensure an effective choice, follow these steps:
- Define Your Analyte: Begin by identifying the chemical nature of your analyte—whether it is polar, non-polar, or ionic—and consider its specific properties. Understanding these traits is crucial for effective distinction.
- Determine Separation Requirements: Establish the resolution and efficiency needed for your analysis. The complexity of the sample matrix must be considered, as this will influence your selection of HPLC stationary phase as the support material.
- Choose the separation mode, selecting the appropriate HPLC stationary phase, such as reversed-phase, normal-phase, or ion-exchange chromatography, based on the characteristics of the analyte. Each mode offers distinct advantages depending on the nature of the compounds involved. As Dr. Avinash Chaudhary notes, "The retention mechanism in RP-HPLC is a complex interplay of hydrophobic interactions, lipophilic interactions, partitioning, and adsorption phenomena," highlighting the intricacies involved in this selection process.
- Consult Manufacturer Specifications: Review the technical data sheets of potential fixed columns. This review will provide insights into their performance metrics, compatibility with your mobile environment, and suitability for your specific application.
- Conduct Preliminary Tests: If feasible, perform small-scale tests using different HPLC stationary phases. This hands-on evaluation will assist in assessing their performance with your analyte and refining your selection.
- Document Your Findings: Maintain detailed records of your selection process and the outcomes of your tests. This documentation will not only aid in refining future analyses but also enhance reproducibility in your laboratory work.
- Consider Temperature and pH: Recent studies have demonstrated that optimizing factors such as temperature and pH can significantly improve efficiency in the process. For instance, the case study titled 'Role of Temperature and pH in High-Performance Liquid Chromatography' illustrates how these elements affect the retention and division of analytes, emphasizing their significance in the selection process.
By adhering to these guidelines, you can optimize your HPLC setup, ensuring that aligns with your analytical goals. Understanding the interplay of various factors will enable you to achieve high separation efficiency and resolution in pharmaceutical applications.
Conclusion
The exploration of High-Performance Liquid Chromatography (HPLC) underscores its critical role in analytical chemistry, particularly within industries such as pharmaceuticals and medical diagnostics. A comprehensive understanding of HPLC necessitates a grasp of its components, notably the significance of stationary phase selection, which directly impacts the efficiency and accuracy of separations. Key factors—including the nature of the analyte, separation goals, mobile phase compatibility, and cost considerations—play integral roles in this selection process.
Diverse stationary phases, ranging from reversed-phase and normal-phase to ion-exchange and chiral phases, cater to specific analytical needs. The ongoing advancements in HPLC technology, particularly in stationary phase innovations, promise to enhance separation efficiency and broaden the applicability of HPLC techniques across various scientific disciplines. As the market for HPLC continues to expand, it is essential for laboratory managers to stay informed about the latest developments and methodologies to optimize their analytical capabilities.
Ultimately, the successful implementation of HPLC hinges on informed decision-making regarding stationary phase selection and a thorough understanding of the unique characteristics of each phase. By applying the outlined strategies and considerations, professionals can significantly improve their analytical outcomes, ensuring compliance with industry standards and the ability to meet the growing demands for precision in analytical processes. Embracing continuous learning in this dynamic field will empower laboratories to leverage HPLC as a powerful tool for achieving high-quality results in complex analytical tasks.




