Overview
This article underscores the critical importance of pH meter calibration solutions, which are vital for ensuring accurate and reliable pH readings in laboratory environments. It highlights that the utilization of standardized buffer solutions, such as pH 4.01, 7.00, and 10.01, is essential for effective calibration. These solutions play a key role in counteracting measurement drift and enhancing the precision of pH meters. Consequently, this directly influences the quality and compliance of laboratory results, emphasizing the necessity of high-quality scientific instruments in professional settings.
Introduction
In the realm of scientific research and laboratory practices, the accuracy of pH measurements is crucial for ensuring reliable outcomes across various applications. pH meter calibration solutions, meticulously designed with defined pH values, form the backbone of this accuracy.
Ranging from the fundamental buffers of pH 4.01 to pH 10.01, these specialized solutions not only calibrate instruments but also mitigate measurement uncertainties that may arise from environmental factors and equipment wear.
As laboratories increasingly acknowledge the significance of regular calibration, comprehending the historical evolution, key characteristics, and market dynamics of these solutions is essential for enhancing operational precision and compliance in a landscape where accuracy is paramount.
Defining pH Meter Calibration Solutions
pH instrument adjustment liquids are essential buffer mixtures with precisely determined pH levels, which serve as a pH meter calibration solution critical for calibrating pH devices to ensure accurate and reliable readings. Commonly utilized standard buffers include:
- pH 4.01
- pH 7.00
- pH 10.01
These represent acidic, neutral, and basic conditions, respectively. The adjustment procedure involves submerging the pH instrument's electrode in these liquids, allowing the device to align its readings with the established values of the buffers. This adjustment is vital for maintaining precision in a variety of scientific applications, including chemistry and medical diagnostics.
The demand for pH meter calibration solution products is significant, as laboratories rely on these solutions to minimize measurement uncertainty, which can be as low as ± 0.02 pH units. Furthermore, the shelf life of these measurement liquids varies; lower pH mixtures typically last 3-6 months, while higher pH mixtures remain effective for 1-3 months after being opened. Understanding this shelf life is crucial for laboratories to manage their resources effectively, as highlighted in the case study 'Shelf Life of pH Measurement Solutions.'
Recent discussions in webinars emphasize the regulatory standards pertaining to pH measurement conformity, highlighting the importance of using certified reference buffer preparations and pH meter calibration solution for consistent adjustment across various pH instruments. As noted by Hanna Instruments, "Whether you are measuring the pH of cheese with or using a benchtop meter in a laboratory setting, standard pH buffers will likely work for you." Practical examples demonstrate that whether evaluating the pH of complex samples or standard laboratory mixtures, employing appropriate reference buffers is crucial for achieving reliable results.
The Importance of Calibration Solutions in Laboratory Accuracy
Calibration methods, such as using a pH meter calibration solution, are essential for maintaining laboratory precision, as they directly impact the reliability of pH readings. Regular adjustments using the pH meter calibration solution are critical to counteract potential drift in pH meter readings caused by factors such as electrode aging, temperature fluctuations, and contamination. can result in significant errors during experiments, affecting vital processes like drug formulation and quality control in pharmaceutical laboratories. Neglecting proper adjustments can compromise product quality and lead to regulatory non-compliance, which poses severe risks for pharmaceutical companies.
A case study highlighting best practices for effective clinical laboratory adjustments underscores the importance of establishing a schedule for these adjustments and conducting regular quality control checks. Such practices ultimately enhance the reliability of diagnostic outcomes and contribute to overall healthcare excellence. Notably, statistics reveal that over 70% of laboratories consistently utilize adjustment fluids, emphasizing their role in maintaining high testing standards. Recent research indicates that using a pH meter calibration solution for routine adjustments can improve precision by as much as 15%, illustrating the tangible benefits of these methods.
Incorporating expert insights makes it clear that the quality of connections between measurement solutions and laboratory practices is crucial. As Charles Eames aptly noted, "Eventually everything connects – people, ideas, objects. The quality of the connections is the key to quality per se." Mastering key parameters such as traceability, adjustment curves, and tests for repeatability, eccentricity, and error of indication is essential for ensuring reliable diagnostics and enhancing patient outcomes. By prioritizing regular adjustments, pharmaceutical labs can significantly bolster their operational accuracy and reliability.
Historical Development of pH Meter Calibration Solutions
The concept of pH was first introduced in 1909 by S.P.L. Sørensen. However, it was Arnold Beckman's development of in the 1930s that marked a pivotal moment in analytical chemistry. Beckman remarked, "I don't dispute with them too vigorously on that," underscoring the significance of this innovation in establishing the necessity for standardized adjustments to ensure precision. Over the years, the evolution of measurement buffers has seen notable advancements, driven by the growing demand for accurate readings across various sectors. Organizations such as the National Institute of Standards and Technology (NIST) have implemented stringent criteria for pH measurement fluids, thereby ensuring consistency and reliability in laboratory practices.
The historical evolution of pH meter adjustment mixtures exemplifies a commitment to accuracy in scientific inquiry. For instance, a uniform 35 kg batch of stoichiometric potassium tetroxalate was created for Standard Reference Material (SRM) 189d, reflecting the meticulous efforts to enhance measurement precision. This development is part of a broader narrative of innovation in adjustment methods that has progressed in tandem with the technology itself.
Moreover, the self-calibrating pH device market is witnessing growth, particularly in portable units, which are favored for their convenience in field applications. According to the "Comprehensive Coverage Self-calibrating pH Meter Report," ongoing innovations in sensor technology and data management are anticipated to drive further market expansion. This underscores the importance of reliable adjustment options in advancing scientific research and industrial applications, especially as the pH instrument market anticipates an increase in demand for pH meter calibration solutions due to the necessity for precise measurements.
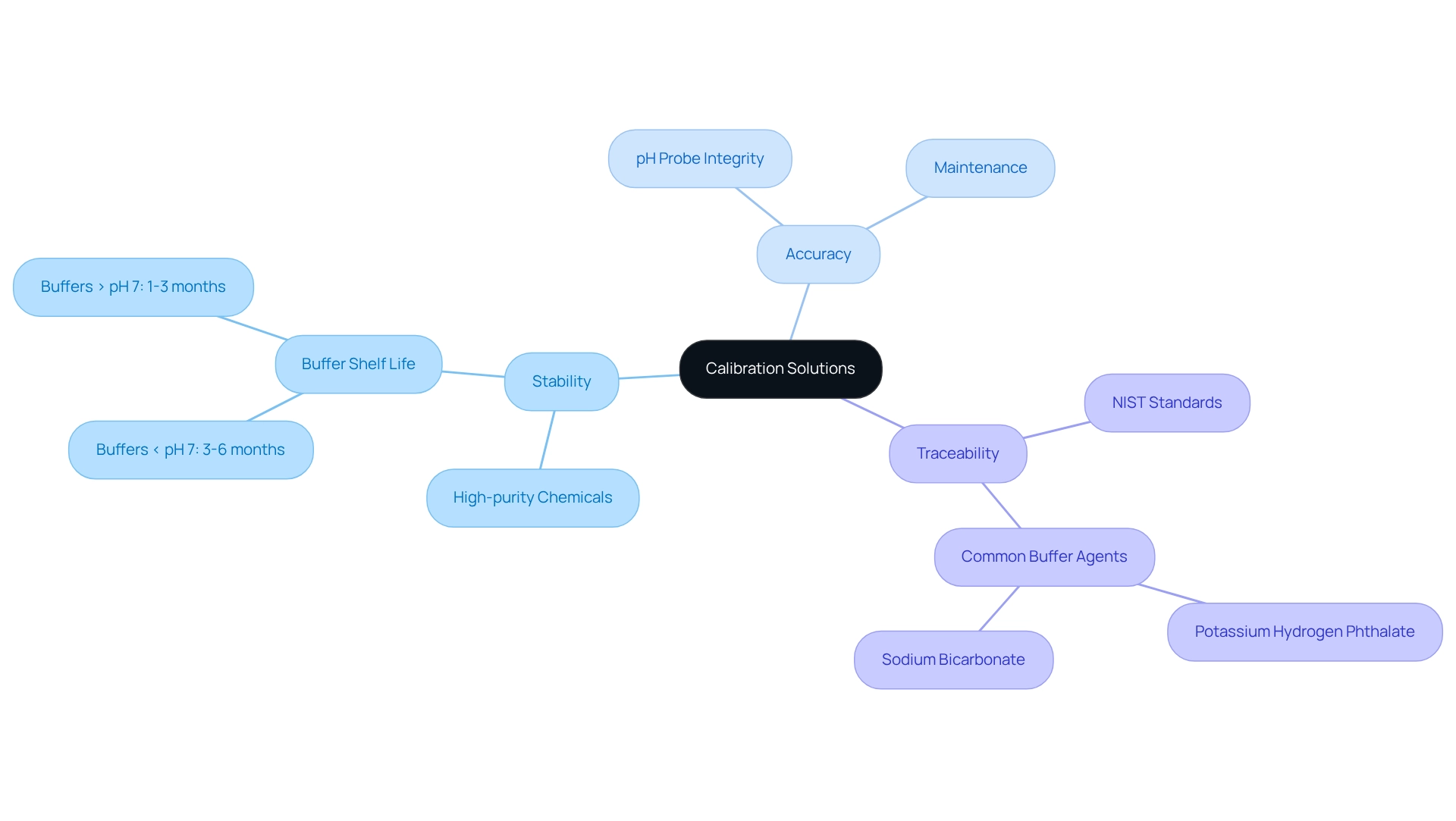
Key Characteristics and Components of Calibration Solutions
Effective pH meter calibration solution methods are characterized by their stability, accuracy, and traceability to recognized standards. These methods must ensure consistent pH levels over time and under varying conditions, achieved through the use of high-purity chemicals. For example, buffers with a pH lower than 7 typically have a shelf life of 3 to 6 months once opened, whereas those above 7 last between 1 to 3 months. This stability is essential for ensuring reliable measurements in laboratory environments.
Traceability to NIST standards is another vital component, as it guarantees the dependability of measurement methods. Common elements of these mixtures include buffer agents such as:
- Potassium hydrogen phthalate for acidic buffers
- Sodium bicarbonate for basic buffers
Additionally, the integrity of significantly impacts accuracy; factors like the cleanliness of the probe and the condition of the reference liquids can greatly influence outcomes. Regular maintenance and checks are crucial to prevent measurement errors and ensure consistent performance.
As Hanna recommends, "If you’d like guidance on the best pH adjustment option for your needs, contact our expert team directly through email or by calling us at 01525 850 855." This underscores the importance of consulting specialists to identify the most suitable options.
Furthermore, JM Science Inc. proudly holds over 780 accreditations across 28 metrological and testing fields, enhancing its expertise and reliability in providing measurement services. In summary, understanding these key characteristics and components empowers laboratories to select appropriate pH meter calibration solutions tailored to their specific measurement needs, ultimately enhancing the reliability of their pH measurements.
Conclusion
The accuracy of pH measurements is non-negotiable in scientific research and laboratory practices. pH meter calibration solutions play a pivotal role in achieving this precision. By utilizing standardized buffers with defined pH values, laboratories can effectively calibrate their instruments, thereby minimizing measurement uncertainties that could jeopardize the integrity of their experiments. The significance of regular calibration cannot be overstated; it directly impacts critical processes such as drug formulation and quality control, ensuring compliance with regulatory standards.
Historically, the development of pH meter calibration solutions has evolved alongside advancements in measurement technology, highlighting a sustained commitment to accuracy and reliability. The meticulous formulation of calibration buffers and adherence to standards set by organizations like the National Institute of Standards and Technology (NIST) underscore the importance of these solutions in maintaining high testing standards across various industries.
Moreover, understanding the key characteristics of calibration solutions—such as stability, traceability, and appropriate components—enables laboratories to select the most suitable options for their specific needs. As the demand for precise pH measurements continues to grow, prioritizing regular calibration with high-quality solutions is essential for enhancing operational accuracy and ensuring reliable scientific outcomes.
In an increasingly complex landscape, where the stakes of measurement accuracy are high, embracing best practices in calibration is critical. By doing so, laboratories not only safeguard the quality of their work but also contribute to the advancement of scientific inquiry and industrial applications. The commitment to precision in pH measurement is a cornerstone of excellence in research and quality assurance, paving the way for reliable results and informed decision-making in the field.




