Overview
To establish a pH lab that ensures pharmaceutical success, it is crucial to meticulously select essential equipment such as:
- pH meters
- Electrodes
- Calibration buffers
- Titration instruments
Compliance with industry regulations and the optimization of lab workflows are equally important. Each component plays a vital role in maintaining the safety and efficacy of pharmaceutical products. Precision and adherence to established standards are not merely recommendations; they are necessities in the pharmaceutical field. By prioritizing these elements, labs can significantly enhance their operational effectiveness and product quality.
Introduction
In the realm of pharmaceutical development, precision and compliance are paramount, particularly when it comes to pH measurement. Establishing a pH lab that meets industry standards necessitates a careful selection of essential equipment and a thorough understanding of regulatory requirements.
From choosing the right pH meters and electrodes to implementing standard operating procedures, every detail plays a crucial role in ensuring the accuracy and reliability of test results. As technology advances, the demand for high-quality instruments and a well-structured lab layout becomes increasingly important.
This article delves into the vital components necessary for setting up an efficient pH lab, sourcing top-notch supplies, and maintaining compliance with stringent regulations. Ultimately, these efforts contribute significantly to the safety and efficacy of pharmaceutical products.
Identify Essential Equipment for a pH Lab
Establishing a pH lab within a drug manufacturing environment requires a meticulous selection of essential instruments to ensure precision and compliance with industry regulations. The significance of medical lab equipment is paramount in safeguarding the safety, efficacy, and quality of pharmaceuticals. Consider the following key components:
- pH Meters: Choose either benchtop or portable models tailored to your laboratory's specific needs. It is critical that the selected meters offer an appropriate range and high precision, as these factors are vital for medical applications.
- pH Electrodes: Opt for electrodes that are compatible with your pH meter and suited for the types of samples you will analyze. Glass electrodes, for example, are ideal for aqueous solutions, delivering reliable readings crucial for drug formulation and quality control.
- Calibration Buffers: Invest in high-quality calibration solutions, specifically pH 4, 7, and 10 buffers. Regular calibration is essential to maintain accuracy in assessments, which is particularly important in medical settings where precision is non-negotiable.
- Sample Containers: Use clean, non-reactive containers for holding samples during testing to prevent contamination and ensure that pH readings accurately reflect the true characteristics of the samples.
- Cleaning Solutions: Maintain appropriate cleaning solutions for electrode upkeep. Proper cleaning is vital to prevent cross-contamination and ensure accurate readings over time.
- Temperature Control Equipment: Since pH measurements can be affected by temperature, consider incorporating a temperature probe or a water bath to maintain consistent sample temperatures during testing.
In addition to these components, the integration of advanced titration equipment, such as the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators, is essential for drug and medicine testing in compliance with the Japanese Pharmacopoeia. The AQV-300 facilitates accurate volumetric analysis, while the AQ-300 excels in coulometric titration, both enhancing the precision of moisture content assessment in pharmaceuticals. These titrators ensure that formulations meet stringent quality benchmarks, making them indispensable in medical applications.
Staying informed about technological advancements that are rapidly reshaping the Lab Equipment Market for the medical field is also critical, as these developments present both opportunities and challenges for manufacturers. Furthermore, compliance with FDA regulations is paramount; utilizing LabX™ software can assist in ensuring adherence to FDA 21 CFR Part 11 regulations, which is essential for maintaining the integrity of drug testing. By assembling these necessary items and considering the regulatory landscape, you lay a robust foundation for a successful pH lab setup, enabling your pH lab to meet the demanding standards of drug testing and support the safety and efficacy of drug development. exemplifies this commitment by fulfilling the needs of pharmaceutical companies, ensuring that their tools and support resources align with industry standards.
Source High-Quality Instruments and Supplies
To source high-quality instruments and supplies for your pH lab, adhere to these essential steps:
- Research Reputable Suppliers: Begin by identifying suppliers renowned for their reliability and quality, such as JM Science, Thermo Fisher Scientific, Agilent Technologies, and Mettler Toledo. These companies are recognized for their commitment to excellence in laboratory tools, including titration instruments like the Hiranuma Aquacounter AQV-300 and AQ-300, which are vital for drug and medicine testing in accordance with the Japanese Pharmacopoeia.
- Check Certifications: It is crucial to verify that the instruments comply with industry standards and certifications, including ISO and FDA regulations, particularly for pharmaceutical applications. Compliance guarantees that the machinery meets the required safety and performance standards.
- Read Reviews and Testimonials: Examine customer feedback and case studies to assess the performance and reliability of the devices. Insights from utilizing can provide valuable context regarding usability and effectiveness. Testimonials from satisfied customers underscore their dependability and exceptional customer service.
- Request Demonstrations: Whenever feasible, arrange for demonstrations of the apparatus to evaluate usability and functionality firsthand. This step is essential for ensuring that the instruments meet your specific laboratory needs.
- Evaluate Warranty and Support: Choose suppliers that offer comprehensive warranties and robust customer support. This ensures assistance with any issues that may arise post-purchase, thereby enhancing your laboratory's operational efficiency.
- Consider Financial Options: For labs facing budget constraints, explore package deals and rental options for lab consumables and equipment. This strategy can help manage costs while still acquiring high-quality instruments.
- Focus on Calibration: When selecting pH meters, ensure they support multiple calibration points, such as the Orion Star A321 pH Portable Meter Kit, which allows for up to a 5-point calibration. This feature significantly improves precision and reliability.
- Use Certified Buffers: Incorporate certified buffers for pH meter calibration to ensure accuracy in your readings. This practice is essential for preserving the integrity of your laboratory findings.
By meticulously selecting top-notch instruments and supplies for your pH lab, including titration systems such as the AQV-300 and AQ-300, you greatly enhance the precision and dependability of your pH assessments, which is crucial for successful drug outcomes.
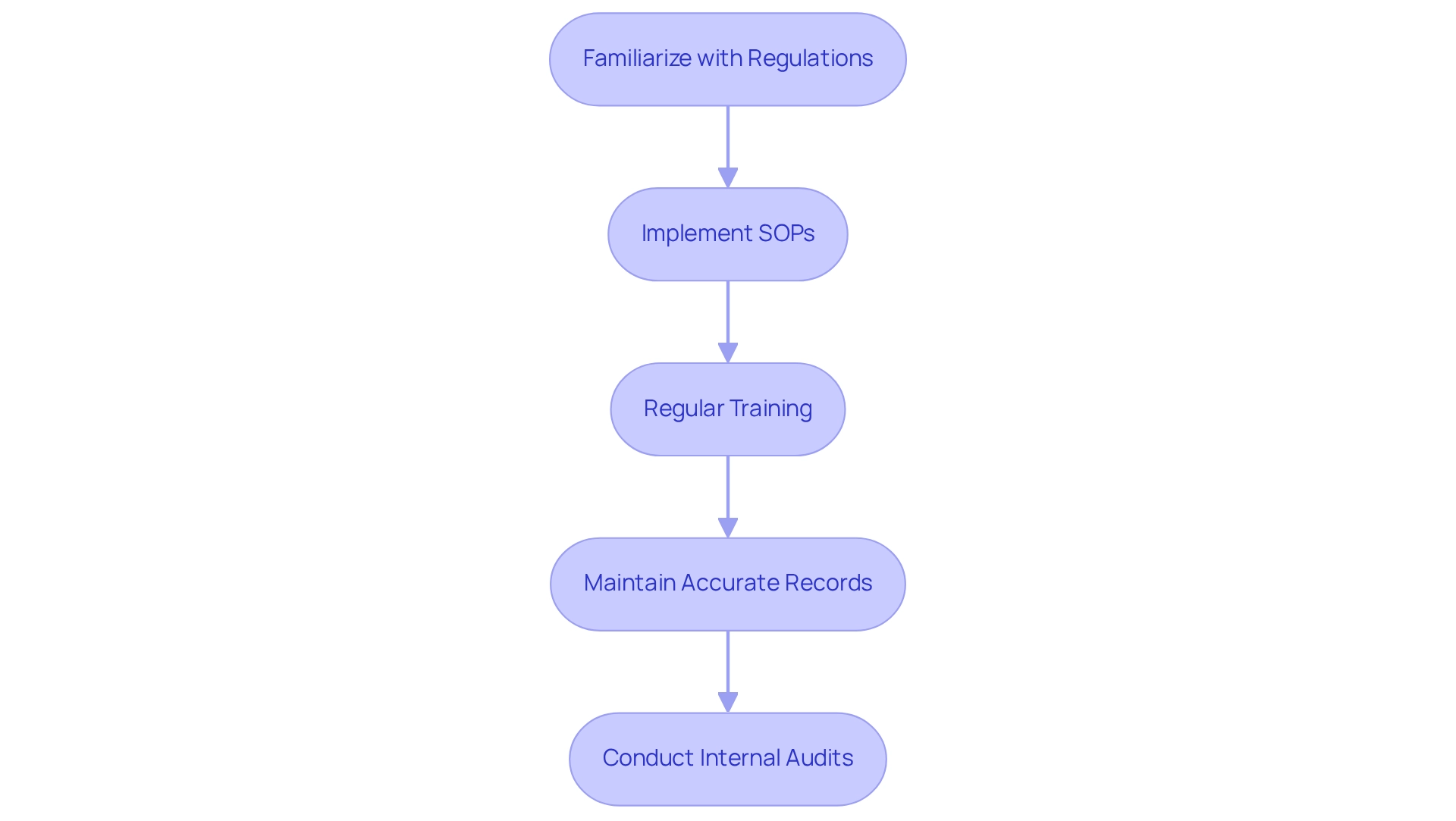
Ensure Compliance with Regulatory Standards
To ensure compliance with regulatory standards in your pH lab, consider the following essential steps:
- Familiarize Yourself with Relevant Regulations: Gain a thorough understanding of applicable regulations, particularly USP <791>, which outlines the requirements for pH assessment in pharmaceuticals. This foundational knowledge is crucial for maintaining compliance and ensuring the integrity of laboratory practices.
- Implement Standard Operating Procedures (SOPs): Create and record detailed SOPs for all pH testing processes. This practice guarantees consistency, accuracy, and adherence to compliance standards across , reinforcing the quality of your operations.
- Regular Training: Arrange continuous training sessions for lab staff to strengthen adherence to compliance standards and optimal practices in the pH lab assessment. Statistics show that effective compliance training can significantly enhance laboratory performance and adherence to standards. This ongoing education is essential for maintaining high standards and ensuring that all staff are up-to-date with current practices.
- Maintain Accurate Records: Keep meticulous records of all pH lab measurements, calibrations, and maintenance activities. Detailed documentation is vital for demonstrating compliance during audits and inspections, serving as a testament to your laboratory's commitment to quality.
- Conduct Internal Audits: Regularly assess lab practices and equipment to ensure continuous compliance with regulatory standards. Internal audits help identify areas for improvement and reinforce a culture of quality and accountability within your laboratory.
By prioritizing these compliance measures, you not only protect your laboratory's operations but also improve the overall quality of medical products, aligning with the industry's commitment to excellence. As noted in the case study "Commitment to Innovation," JM Science continually updates its product offerings to support laboratories in achieving these standards. Dr. Peter Mere Latham emphasizes, "The foundation of quality in laboratory practices lies in rigorous adherence to established protocols and continuous improvement.
Establish an Efficient Lab Layout and Workflow
To establish an efficient lab layout and workflow for pH measurement in pharmaceutical settings, it is essential to consider the following guidelines:
- Designate Specific Areas: Allocate distinct zones for sample preparation, pH measurement, and data analysis. This separation minimizes cross-contamination and enhances process efficiency. For instance, Bassett Health Network's redesign emphasized clear area delineation, contributing to significant efficiency gains.
- Optimize Instrument Arrangement: Position essential tools, such as pH meters and calibration stations, within easy reach. This strategic placement reduces unnecessary movement, saving valuable time during operations. The redesign at Bassett Health Network also concentrated on optimal placement of tools, which was crucial for their workflow enhancements.
- Implement a Logical Workflow: Create a step-by-step process that guides users through the pH assessment procedure, from sample collection to data recording. A well-structured workflow not only improves accuracy but also enhances user confidence. As noted by Bassett Health Network, their strategic approach resulted in increased efficiencies, including a 65% reduction in process steps.
- Ensure Adequate Storage: Provide ample storage for reagents, buffers, and samples to maintain an organized and clutter-free workspace. An orderly environment is crucial for efficient lab operations. This aspect was a key focus in the redesign, ensuring that all materials were easily accessible and well-organized.
- Incorporate Safety Measures: Ensure that safety equipment, such as eyewash stations and fire extinguishers, are easily accessible within the lab layout. Prioritizing safety is essential for compliance and the well-being of lab personnel. The integration of safety measures is a fundamental component of , aligning with best practices in sustainable resource management.
By concentrating on these elements, pH labs can significantly enhance productivity and accuracy in measurement operations. Emphasizing effective space design and technological integration can further streamline processes, ultimately contributing to improved outcomes in pharmaceutical research and diagnostics. As Bassett Health Network stated, "The strategic approach resulted in increased efficiencies that included a 71% reduction in touchpoints," highlighting the profound impact of optimized workflows on laboratory efficiency.
Conclusion
Establishing a pH lab that meets the rigorous demands of pharmaceutical development is essential for ensuring the safety and efficacy of drug products. High-quality pH meters, compatible electrodes, and reliable calibration buffers form the foundation for accurate measurements. Moreover, sourcing supplies from reputable suppliers and ensuring compliance with industry regulations enhance the lab's operational integrity, which is paramount in the pharmaceutical sector.
The significance of adhering to regulatory standards cannot be overstated. Implementing standard operating procedures, maintaining meticulous records, and conducting regular training and audits are vital practices that bolster compliance and ensure high-quality outcomes. An efficient lab layout and workflow are also crucial in optimizing performance, reducing errors, and fostering a culture of safety and precision.
In conclusion, the collective efforts to establish a well-equipped, compliant, and efficiently organized pH lab significantly contribute to the overarching goals of pharmaceutical development. By prioritizing these elements, laboratories enhance their operational effectiveness and uphold their commitment to delivering safe and effective pharmaceutical products, ultimately benefiting public health and safety.




