Overview
This article serves as a comprehensive tutorial on mastering coulometric Karl Fischer titration, underscoring its critical role in accurately measuring water content across various substances, with a particular focus on the pharmaceutical industry. It elucidates the underlying principles, delineates the distinctions between coulometric and volumetric methods, and outlines essential factors for selecting the most suitable approach. Furthermore, the article provides detailed, step-by-step procedures for executing the titration, reinforcing its significance in maintaining product quality and ensuring compliance with regulatory standards.
Introduction
Karl Fischer titration stands as a cornerstone of analytical chemistry, esteemed for its precision in determining moisture content across various industries, particularly pharmaceuticals. This tutorial explores the intricacies of coulometric Karl Fischer titration, providing readers with a comprehensive understanding of its principles, methods, and practical applications. Given the distinct advantages and challenges presented by the choice between coulometric and volumetric methods, it is crucial for laboratories to ensure they select the optimal approach tailored to their specific needs.
Understand the Principles of Karl Fischer Titration
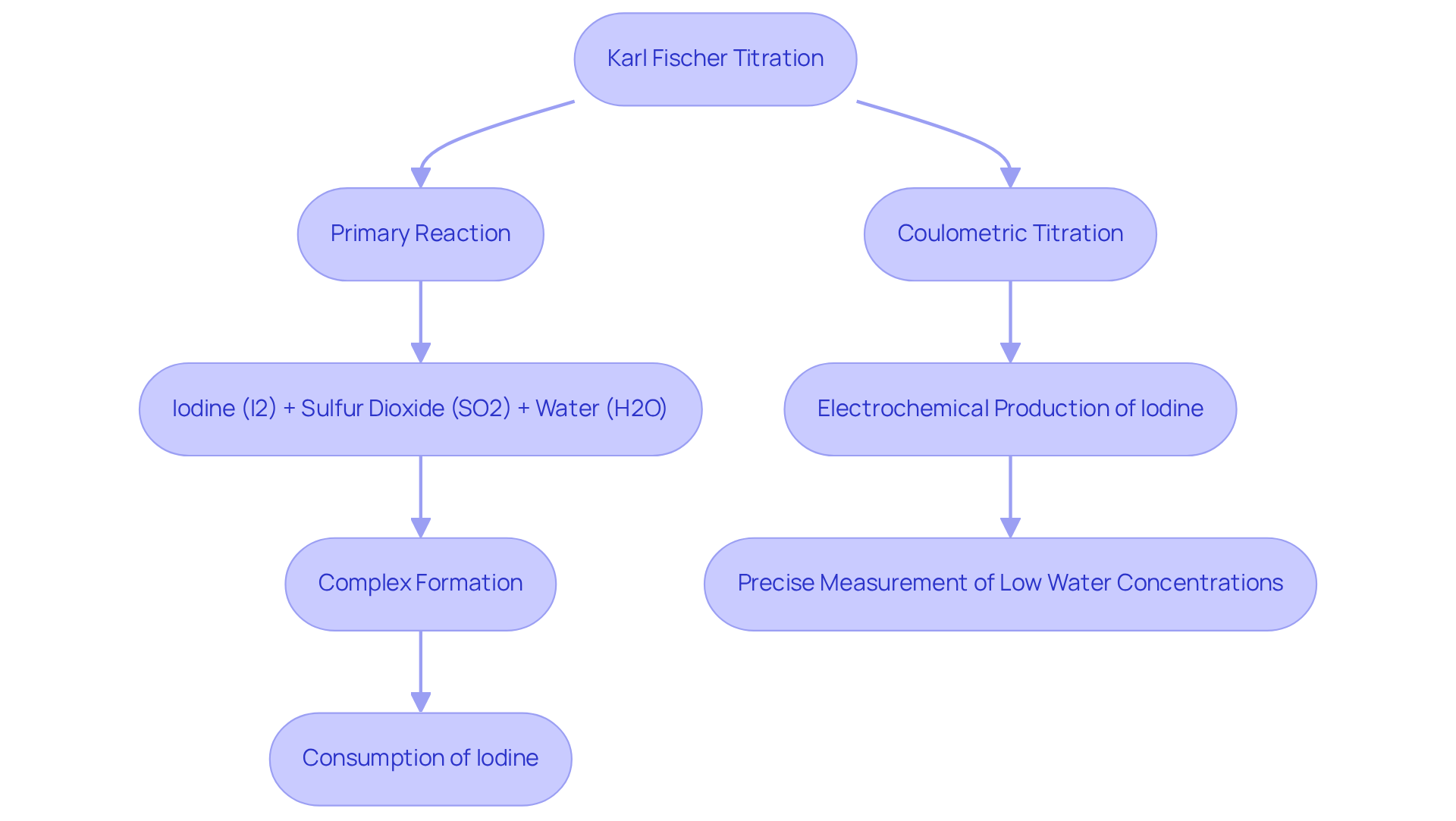
Karl Fischer analysis stands as a fundamental analytical method, essential for precisely assessing water levels in various substances, particularly within the pharmaceutical industry. This method is predicated on a where iodine interacts with water in the presence of sulfur dioxide and a base. The chemical reaction can be summarized as follows:
- The primary reaction involves iodine (I2) and sulfur dioxide (SO2) reacting with water (H2O) to form a complex that ultimately leads to the consumption of iodine, facilitating accurate humidity measurement.
- In the realm of [coulometric Karl Fischer titration](https://jmscience.com/collections/karl-fischer-titrators?srsltid=AfmBOop-id2_Lhi1yFZQtmrdllHEaznv8uM0bKyg-GBKS327-mNy4kQ4), iodine is produced electrochemically, enabling precise measurement of low water concentrations, particularly in samples with water content under 1%. This level of accuracy is crucial for pharmaceutical applications, where even slight variations in humidity can significantly impact product stability and efficacy.
Karl Fischer analysis finds extensive application across the pharmaceutical, food, and chemical industries, ensuring product quality and compliance with stringent regulatory standards. Notably, approximately 70% of laboratories utilize Karl Fischer analysis for water content examination, underscoring its vital role in maintaining product integrity and safety. As the demand for accurate moisture content determination continues to rise, particularly within the pharmaceutical sector, the relevance of coulometric Karl Fischer titration remains paramount.
Differentiate Between Coulometric and Volumetric Methods
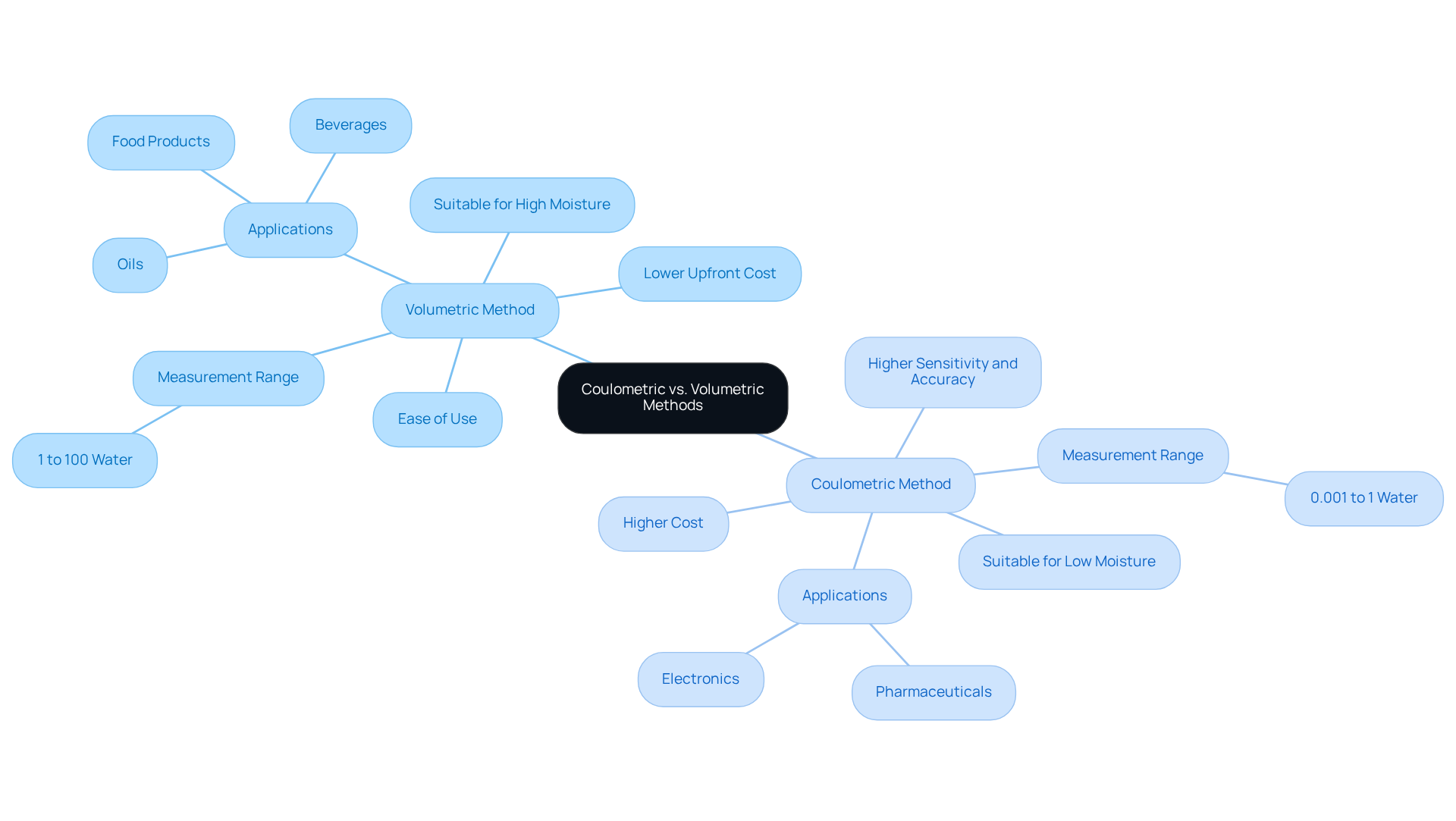
Coulometric Karl Fischer titration is a critical technique in analytical chemistry, which can be performed using two primary methods: volumetric and coulometric.
- Volumetric Method: This method involves the addition of a to the sample until the endpoint is reached. It is particularly suitable for specimens with elevated humidity levels, typically exceeding 1%. The titrant, generally a solution of iodine in a solvent, facilitates operation, especially in routine high-moisture testing scenarios. Volumetric KF is favored for high-moisture materials such as beverages, oils, and food items, where ease of use is paramount.
- Coulometric Method: Conversely, the coulometric method generates iodine in situ through an electrochemical reaction, making it ideal for specimens with low water content, usually below 1%. This technique offers enhanced sensitivity and accuracy for trace water detection, which is essential in fields requiring precise measurements, such as pharmaceuticals and electronics. Coulometric Karl Fischer titration is specifically designed for trace water analysis, with a measurement range from 0.001% to 1%.
- Comparison: While volumetric analysis is simpler to conduct for larger samples, coulometric measurement excels in precision for smaller samples and lower humidity levels. Laboratories frequently face the challenge of choosing between these methods based on their specific water content requirements. Current adoption trends indicate a growing preference for coulometric Karl Fischer titration in sensitive applications, showcasing its precision in moisture measurement. Laboratory managers have observed that the choice between volumetric and coulometric methods significantly influences the reliability of their analyses, especially in environments where trace moisture detection is vital. Selecting the appropriate Karl Fischer method is essential for accurate water content assessment. Understanding these distinctions is crucial for making informed decisions in your analytical processes.
Select the Appropriate Titration Method for Your Application
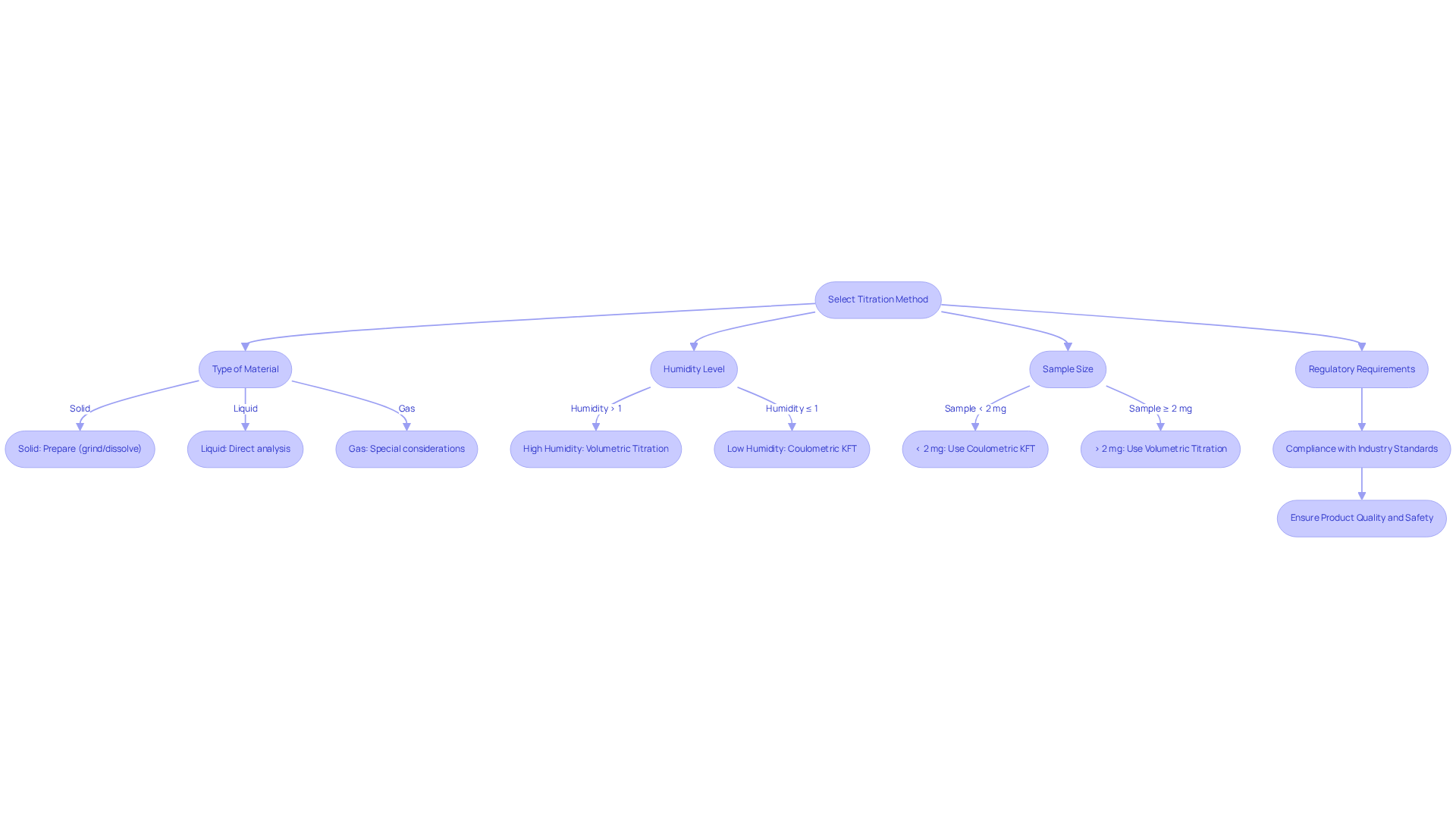
Selecting the appropriate titration method in pharmaceutical laboratories is a critical decision that demands careful consideration of several key factors.
- Type of Material: First, determine whether your material is solid, liquid, or gas. Solid specimens often necessitate specific preparation methods, such as grinding or dissolving, to ensure precise results.
- Humidity Level: Next, assess the anticipated humidity level of your specimen. For instance, volumetric titration is typically employed for specimens with water levels exceeding 1%, whereas coulometric Karl Fischer titration is the preferred method for those with lower water levels. This distinction is crucial, as moisture levels can significantly impact the . Studies indicate that the water content of various bread types can range from 35.1% to 44.2%, with water reaction rates for 'strongly retained' water molecules in whole meal and brown bread examples varying from 0.32 mM/s to 0.44 mM/s. Such variability must be meticulously accounted for in method selection.
- Sample Size: Additionally, evaluate the quantity or weight of your specimen. Coulometric Karl Fischer titration excels with smaller samples, often less than 2 mg, while volumetric methods are suitable for larger quantities, providing versatility to meet diverse laboratory needs.
- Regulatory Requirements: Finally, ensure that the selected method complies with industry standards and regulations relevant to your field, such as those established for pharmaceuticals and food safety. Pharmaceutical lab managers underscore the importance of adhering to these regulations to uphold product quality and safety. Notably, the registered specification limit of NMT 1.0% w/w is significant for regulatory compliance in pharmaceutical settings.
By considering these factors, laboratories can refine their measurement processes, ensuring accurate and reliable results that align with both scientific and regulatory standards.
Execute a Coulometric Karl Fischer Titration: A Step-by-Step Guide
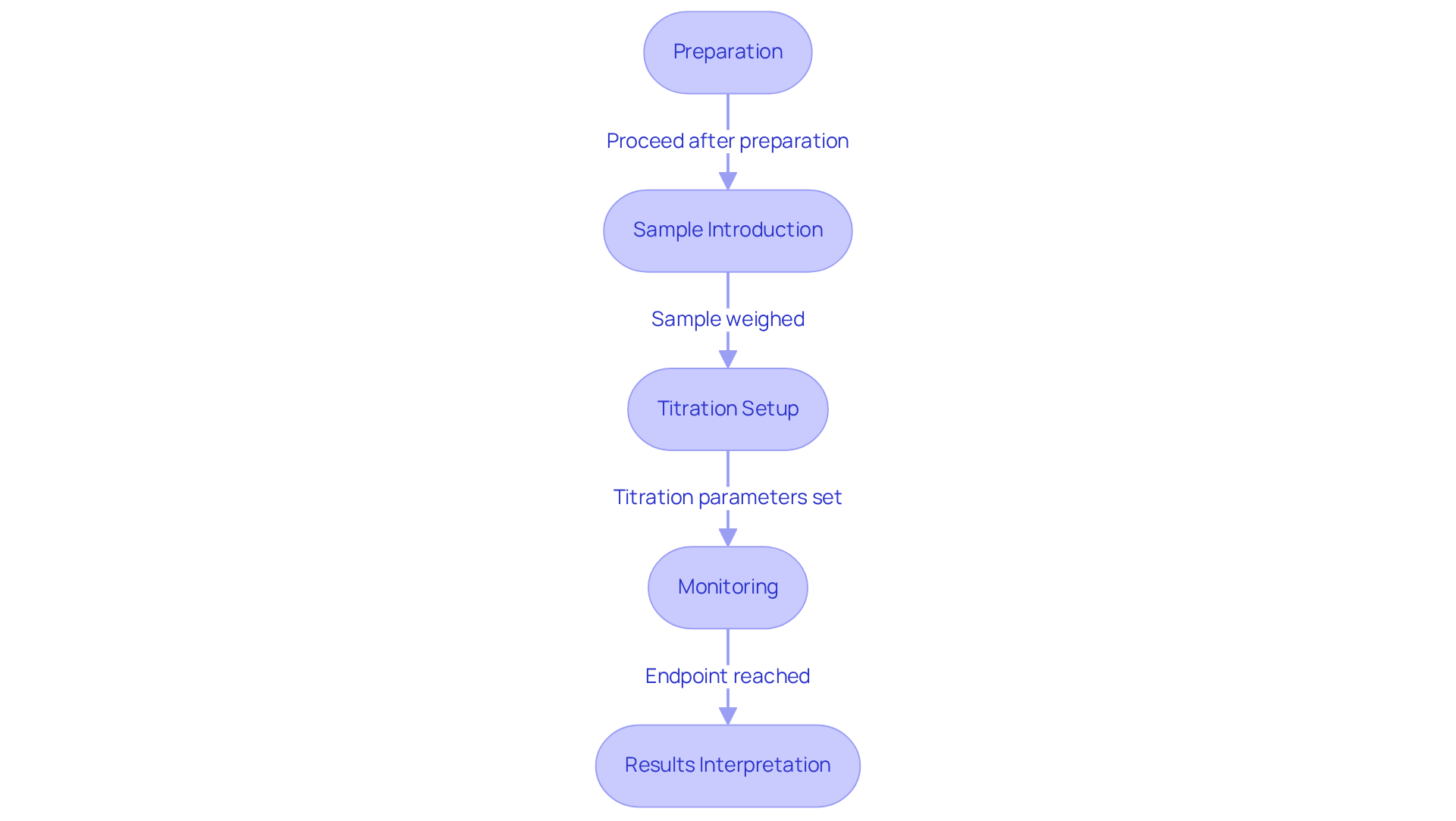
To successfully perform a coulometric Karl Fischer titration, it is essential to follow these detailed steps:
- Preparation: Ensure the titrator is properly calibrated and functioning optimally. Regular calibration, ideally on a monthly basis, is crucial for accurate results. Additionally, check the KF Factor daily to maintain precision in measurements. Prepare the titration cell by filling it with the appropriate solvent, typically methanol, and the Karl Fischer reagent. Ensure all glassware is clean and dry to avoid contamination.
- Sample Introduction: Accurately weigh the sample, ensuring it is representative of the batch being analyzed. This step is essential, as an incorrect weight of the specimen can result in considerable inaccuracies in water measurement. Make certain that the specimen is devoid of pollutants and that the measuring container is sealed during the procedure to improve precision.
- Titration Setup: Configure the on the instrument, including the sample size and expected moisture content. Setting these parameters correctly is vital for achieving precise results. Begin the process, enabling the instrument to produce iodine as required. Monitor the system closely to ensure it operates within the defined parameters.
- Monitoring: Continuously observe the titration process on the display. The endpoint is reached when the current stabilizes, indicating that all water has reacted. This confirmation typically occurs within a 20-second timeframe.
- Results Interpretation: Record the results displayed by the titrator, calculating the moisture content based on the amount of iodine consumed during the titration. Accurate documentation of all relevant data is essential for compliance and quality control purposes.
Best Practices: Implementing proper sample preparation techniques is crucial for accurate water content determination. Ensure that the specimen is free from impurities and that the measurement container is airtight during the process. Common mistakes in coulometric Karl Fischer titration include miscalibrated instruments and incorrect handling of materials, which can occur frequently if not monitored closely. Laboratory technicians often report challenges such as maintaining consistent environmental conditions, which can affect results.
Real-World Examples: Successful coulometric Karl Fischer titration procedures have been documented in various pharmaceutical labs, illustrating the method's reliability in determining moisture content in active pharmaceutical ingredients (APIs). For instance, adjusting the Karl Fischer technique to particular sample categories has resulted in enhanced precision in water content analysis, demonstrating the method's adaptability across various laboratory environments.
By adhering to these guidelines and best practices, laboratories can significantly enhance the accuracy and reliability of their moisture content determinations through coulometric Karl Fischer titration.
Conclusion
Mastering coulometric Karl Fischer titration is not merely advantageous; it is essential for laboratories striving for precise moisture content analysis. This analytical technique, rooted in a redox reaction, offers unparalleled accuracy, which is particularly critical in industries such as pharmaceuticals, where water levels can significantly affect product stability. By understanding the principles and applications of this method, laboratories empower themselves to ensure compliance with stringent quality standards.
The distinctions between coulometric and volumetric methods have been clearly highlighted, underscoring the necessity of selecting the appropriate technique based on the sample's water content and material type. Key factors—including humidity levels, sample size, and regulatory requirements—are pivotal in determining the most effective titration approach. By adhering to a structured step-by-step guide, laboratories can optimize their procedures to achieve accurate results.
Ultimately, the importance of mastering coulometric Karl Fischer titration transcends mere compliance; it is fundamentally about safeguarding the integrity and safety of products that depend on precise moisture measurements. Laboratories are strongly encouraged to adopt best practices and remain informed about advancements in titration techniques, enhancing their analytical capabilities. Embracing this knowledge not only boosts operational efficiency but also significantly contributes to overall quality assurance in critical sectors.




