Overview
Coulometric KF titration stands as a highly accurate method for measuring low moisture content across various industries, particularly within pharmaceuticals, petrochemicals, and food production. This technique demonstrates exceptional effectiveness, achieving precision levels between 102% and 103%, accompanied by low relative standard deviations. Such accuracy is crucial for ensuring product quality and stability, where even minute variations in moisture can significantly impact outcomes. Therefore, the adoption of this method is essential for industries that prioritize precision and reliability in their processes.
Introduction
Coulometric Karl Fischer titration represents a pinnacle in analytical chemistry, delivering unmatched precision in quantifying moisture content across diverse industries. This advanced technique not only produces its own titrant but also demonstrates exceptional capability in detecting moisture levels as low as 1 ppm. As such, it has become an indispensable instrument in sectors such as pharmaceuticals, petrochemicals, and food production.
Nonetheless, despite its numerous advantages, practitioners face challenges in its application, particularly with high moisture samples. What critical principles and practices can enhance the efficacy of this method, and how does it measure up against traditional volumetric titration?
Explore the Fundamentals of Coulometric Karl Fischer Titration
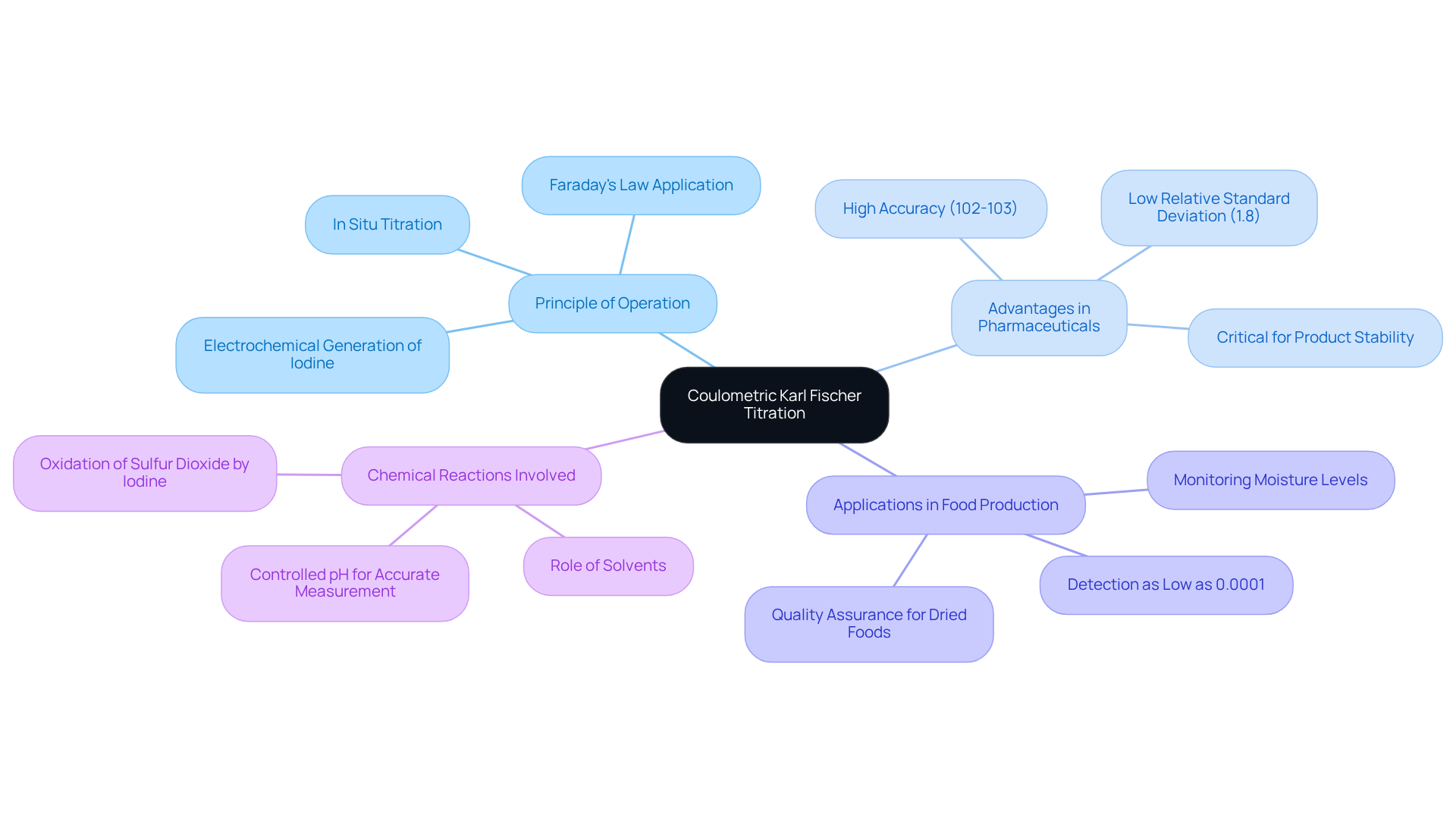
[Coulometric KF titration](https://jmscience.com) is a highly accurate analytical method designed to quantify minute quantities of moisture in various materials. This sophisticated approach relies on the , which interacts directly with the liquid present in the specimen. In contrast to volumetric analysis, which utilizes a pre-prepared standard solution, coulometric KF titration methods generate the titrant in situ. This capability enables precise measurements of very low water content, typically ranging from 1 ppm to 5%. Such precision renders coulometric KF titration particularly advantageous for samples with minimal moisture, making it an indispensable tool in industries such as pharmaceuticals, petrochemicals, and food production.
The advantages of using coulometric KF titration in pharmaceutical applications are profound. It facilitates accurate assessments of moisture content, a critical factor in ensuring product stability and quality. Recent research underscores that coulometric KF titration can achieve accuracy levels between 102% and 103%, with a relative standard deviation as low as 1.8%, particularly when employing reagents with high concentrations of imidazole and modifiers like hexanol and chloroform. This level of accuracy is essential in pharmaceutical production, where even minute quantities of moisture can significantly impact the effectiveness and safety of products.
In the realm of food production, coulometric KF titration is employed to monitor moisture levels across various products, ensuring adherence to quality standards. The method's capacity to detect moisture content as low as 0.0001% proves invaluable for assessing the quality of dried foods and other moisture-sensitive items.
A comprehensive understanding of the underlying chemical reactions, including the consumption of iodine and the role of solvents, is vital for the effective application of this technique. By mastering these principles, laboratories can enhance their analytical capabilities, ensuring reliable results that meet the stringent demands of modern industries.
Understand the Operational Principles of Coulometric Titration
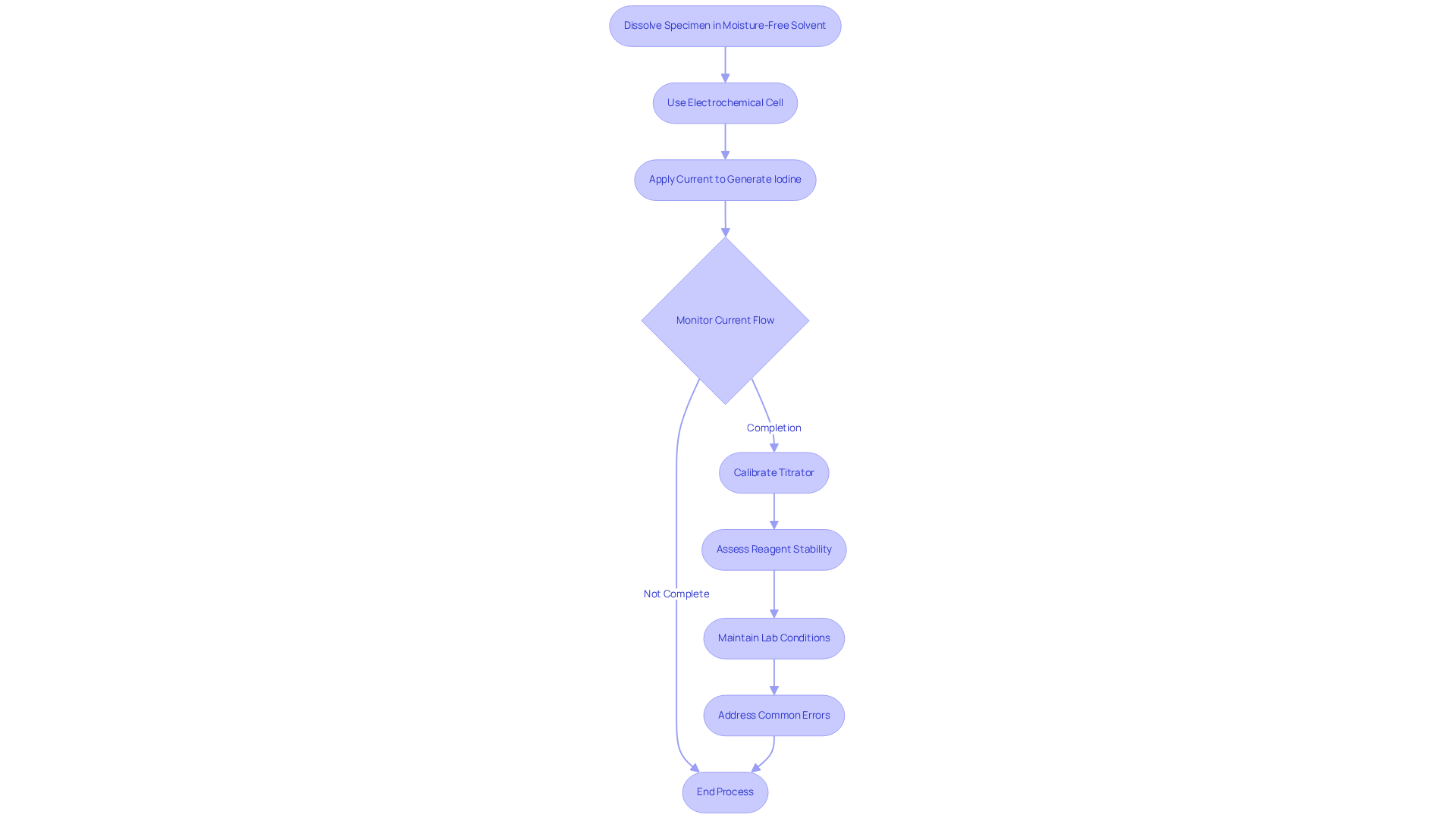
Coulometric kf titration operates on the principle of quantifying the electric charge required to fully react with the liquid present in the specimen. The procedure commences with dissolving the specimen in a moisture-free solvent, typically methanol. An electrochemical cell, comprising an anode and a cathode, is then employed. Upon applying a current, iodine is generated at the anode, subsequently interacting with the liquid in the sample. The process concludes when the current flow indicates that all the liquid has reacted. This method is distinguished by its high sensitivity and accuracy, making it particularly suitable for applications demanding precise moisture content determination. Coulometric analysis can measure water levels significantly lower than volumetric methods, underscoring its precision in laboratory environments.
To achieve reliable measurements, proper calibration of the coulometric titrator is crucial. Regular assessments of reagent stability and the electrochemical cell's condition can markedly enhance measurement reliability. Contemporary titrators frequently incorporate automated systems that monitor reagent quality and issue alerts for expired materials, thereby preserving the integrity of results.
Recent studies have validated the efficacy of coulometric titration across various laboratory contexts. For instance, the AQ-2200AF trace moisture content analyzer has exhibited outstanding performance in quantifying humidity levels in diverse materials, including chemical products and pharmaceuticals. The average moisture content of 154 samples of dried honeybee-collected pollen ranged from 3% to 9%, illustrating the analyzer's efficiency in practical applications. Its single-chamber electrolysis method simplifies maintenance and lowers operational costs, rendering it a favored choice in numerous laboratories.
Current trends in electrochemical analysis underscore the growing adoption of automated systems that streamline workflows and enhance precision. Understanding how functions in coulometric kf titration is vital, as it allows for accurate calculations of water content based on the current required for iodine production. Moreover, maintaining laboratory conditions—such as keeping the temperature between 20°C and 25°C and relative humidity below 50%—is critical for ensuring accurate results in coulometric kf titration. Common errors, including atmospheric humidity interference and improper sample handling, should be systematically addressed to mitigate challenges in the process. This accuracy is paramount in sectors where humidity levels can significantly impact product quality and stability.
Identify Key Applications of Coulometric KF Titration in Industry
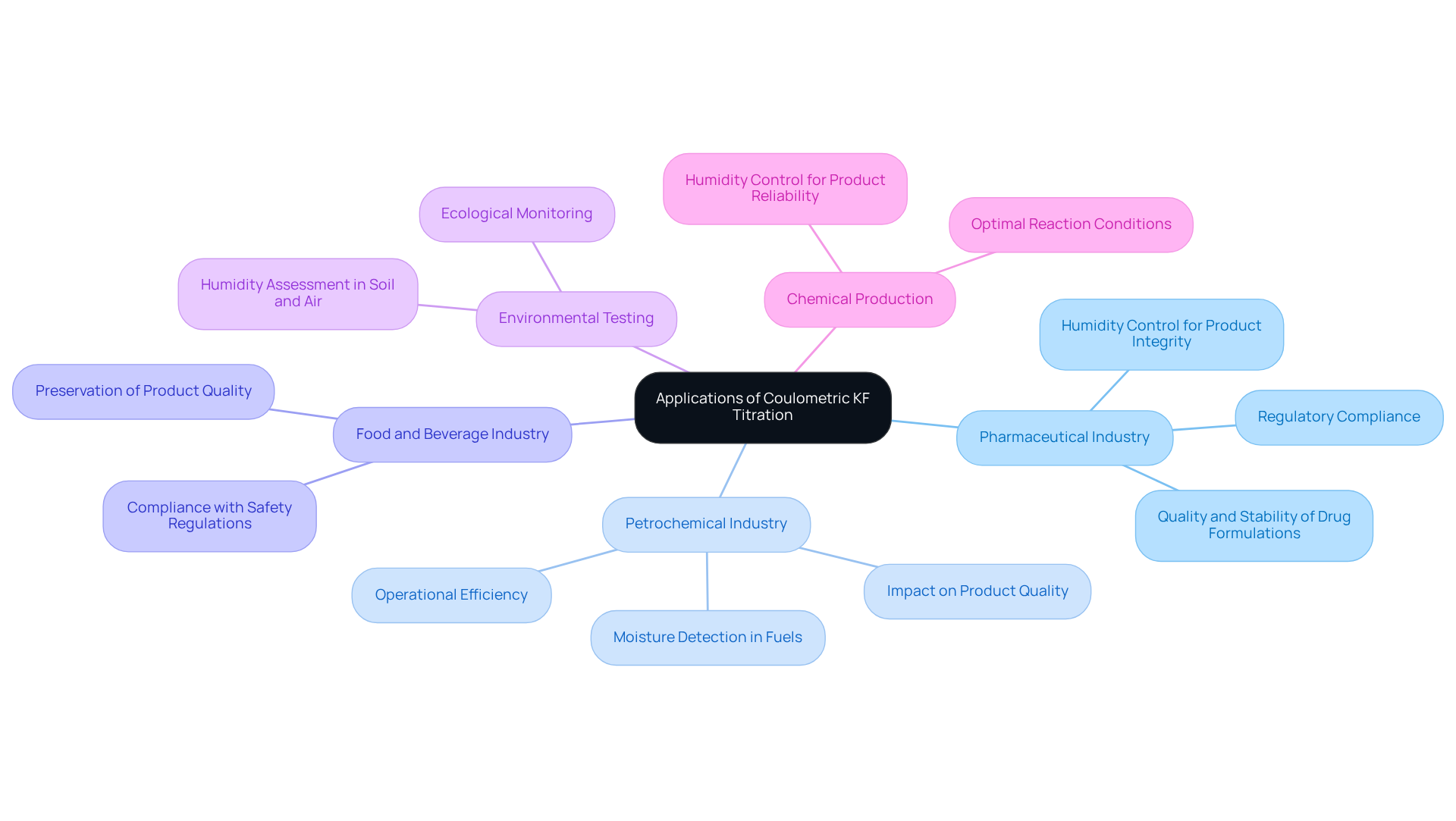
Coulometric KF titration is a pivotal analytical technique utilized across various industries, renowned for its precision in quantifying low water content. In the pharmaceutical sector, it is indispensable for ensuring the quality and stability of drug formulations, as humidity levels are critical for the integrity, quality, and efficacy of pharmaceutical products. Even minor fluctuations in humidity can profoundly affect product performance, with accurate measurements safeguarding their reliability.
In the petrochemical industry, coulometric KF titration is crucial for assessing water content in fuels and lubricants, which directly impacts product quality and operational efficiency. The method's ability to detect humidity levels as low as a few parts per million (ppm) is particularly beneficial, as excessive moisture can lead to significant operational challenges and product degradation. Recent studies underscore that maintaining humidity levels within specified limits is vital for optimizing the performance of petrochemical products, highlighting the need for reliable humidity analysis. A modified procedure has notably achieved a 99.4% recovery rate for petroleum products, further demonstrating the method's efficacy.
Moreover, the food and beverage industries leverage coulometric KF titration to monitor water content in various products, ensuring compliance with safety regulations and preserving product quality. This technique is also employed in environmental testing, where it in soil and air samples, facilitating ecological monitoring and research.
The versatility of coulometric KF titration extends to chemical production, where precise humidity control is essential for achieving optimal reactions. As industries increasingly recognize the importance of humidity management, the demand for accurate and effective humidity assessment techniques, such as coulometric analysis, continues to grow.
Evaluate Advantages and Limitations of Coulometric vs. Volumetric Titration
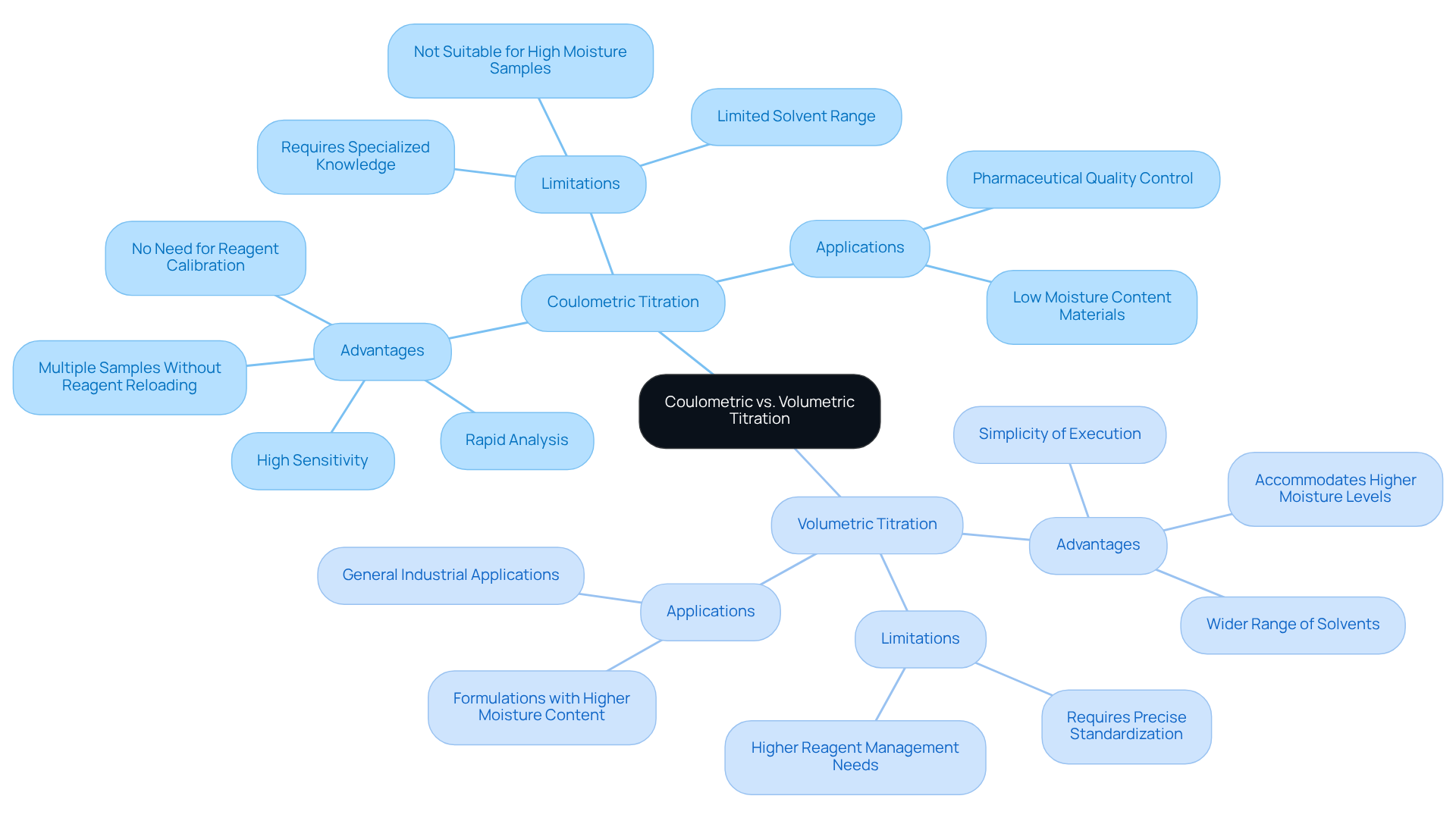
Coulometric kf titration and volumetric analyses are essential methods for assessing moisture levels, with each method tailored to the specific characteristics of the material. Coulometric analysis excels with low moisture materials, typically preferred for moisture levels below 1%. It can detect liquid content as minimal as 1 microgram. This method generates the titrant on-site, facilitating accurate measurements without the need for standard solutions, making it particularly advantageous for small or difficult-to-dissolve specimens. The ideal specimen for coulometric analysis contains less than 1% moisture and is limited to 2 milliliters, yielding under 20 micrograms of water.
However, coulometric analysis has notable limitations, especially with high humidity samples. When water content exceeds 1%, particularly beyond 2%, the reagent capacity may be compromised, leading to erroneous results. In such cases, volumetric analysis is the preferred technique, accommodating humidity levels from 1% to 100% by allowing larger quantities of reagent to be utilized. While volumetric analysis is generally simpler to execute, it necessitates , which can introduce variability in the results.
Real-world applications underscore these distinctions: for example, in pharmaceutical laboratories, coulometric kf titration is frequently employed for raw materials with low water content, ensuring accurate quality control. Conversely, volumetric titration is used for formulations with higher moisture levels, where the capacity to add larger titrant volumes is beneficial. Recognizing these differences is crucial for selecting the appropriate method to fulfill specific analytical requirements.
Conclusion
Coulometric Karl Fischer titration emerges as a pivotal analytical method for accurately measuring minute moisture levels across diverse industries. By generating the titrant in situ, this technique delivers unmatched precision, especially for samples with low water content. Its application is indispensable in sectors such as pharmaceuticals, petrochemicals, and food production, where even minor moisture fluctuations can profoundly influence product quality and stability.
This article explores the operational principles of coulometric titration, underscoring the necessity of precise calibration and the contribution of automated systems in enhancing measurement reliability. Key applications illustrate how this method not only fulfills stringent industry standards but also aids in environmental monitoring and chemical production. A comparison with volumetric titration highlights the strengths and limitations of each approach, guiding users in selecting the most suitable method for their specific analytical requirements.
As industries increasingly prioritize moisture control, the significance of coulometric KF titration will continue to escalate. Adopting this technique can yield enhanced product quality and operational efficiency, making it vital for both laboratories and manufacturers. By comprehending the intricacies of coulometric KF titration, professionals can fully leverage its potential, ensuring reliable results in an ever-evolving landscape.




