Overview
Mastering titrimetry in pharmaceutical labs is essential for ensuring precise measurement techniques, selecting appropriate titration methods, and maintaining rigorous quality control and regulatory compliance.
A thorough understanding of titration fundamentals is paramount. Adhering to proper calibration and measurement practices, alongside implementing strict quality management systems, is vital for achieving reliable results.
Such diligence not only upholds high standards in pharmaceutical analysis but also reinforces the credibility of laboratory practices.
Introduction
Titrimetry serves as a foundational element in pharmaceutical analysis, facilitating the precise determination of substance concentrations through meticulous chemical reactions. By mastering this quantitative technique, laboratories can significantly enhance their operational accuracy and ensure compliance with regulatory standards. However, navigating the complexities of various titration methods presents a challenge, necessitating consistent measurement practices.
What best practices can pharmaceutical labs adopt to optimize titrimetry and uphold the highest quality control?
Understand Titrimetry Fundamentals
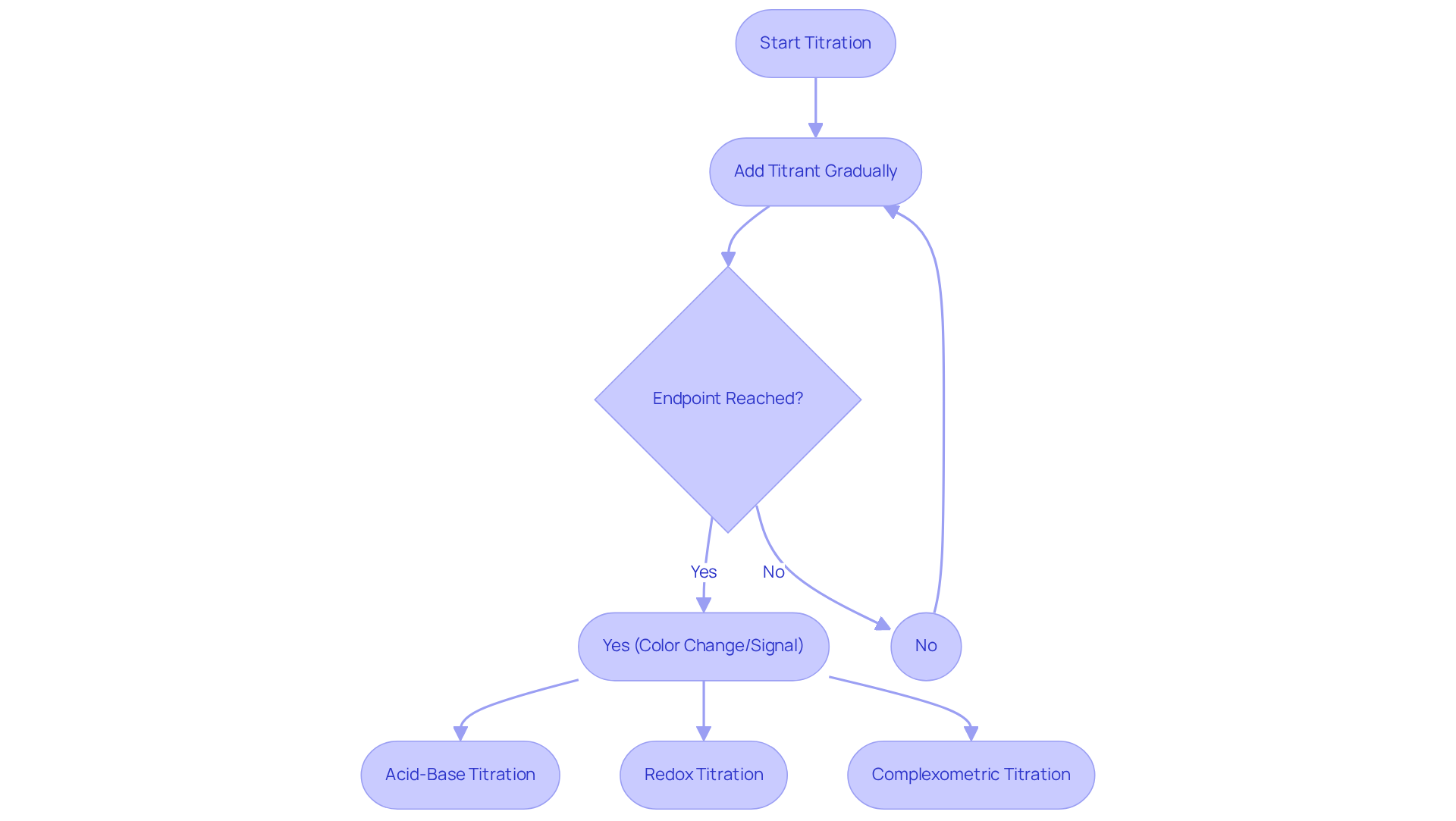
Titrimetry is a crucial quantitative analytical technique used for determining the concentration of a specific analyte. This method involves the careful and gradual addition of a titrant to a solution containing the analyte until the reaction reaches its endpoint, which is typically indicated by a color change or an electrical signal.
Understanding the various types of titrations—such as acid-base, redox, and complexometric—is crucial for selecting the appropriate for specific applications. Furthermore, a solid grasp of the fundamental chemical reactions and stoichiometry involved in titrimetry is essential for conducting precise calculations and accurately interpreting findings.
This foundational knowledge empowers laboratory staff to resolve problems efficiently and enhances the overall measurement processes.
Implement Accurate Measurement Techniques
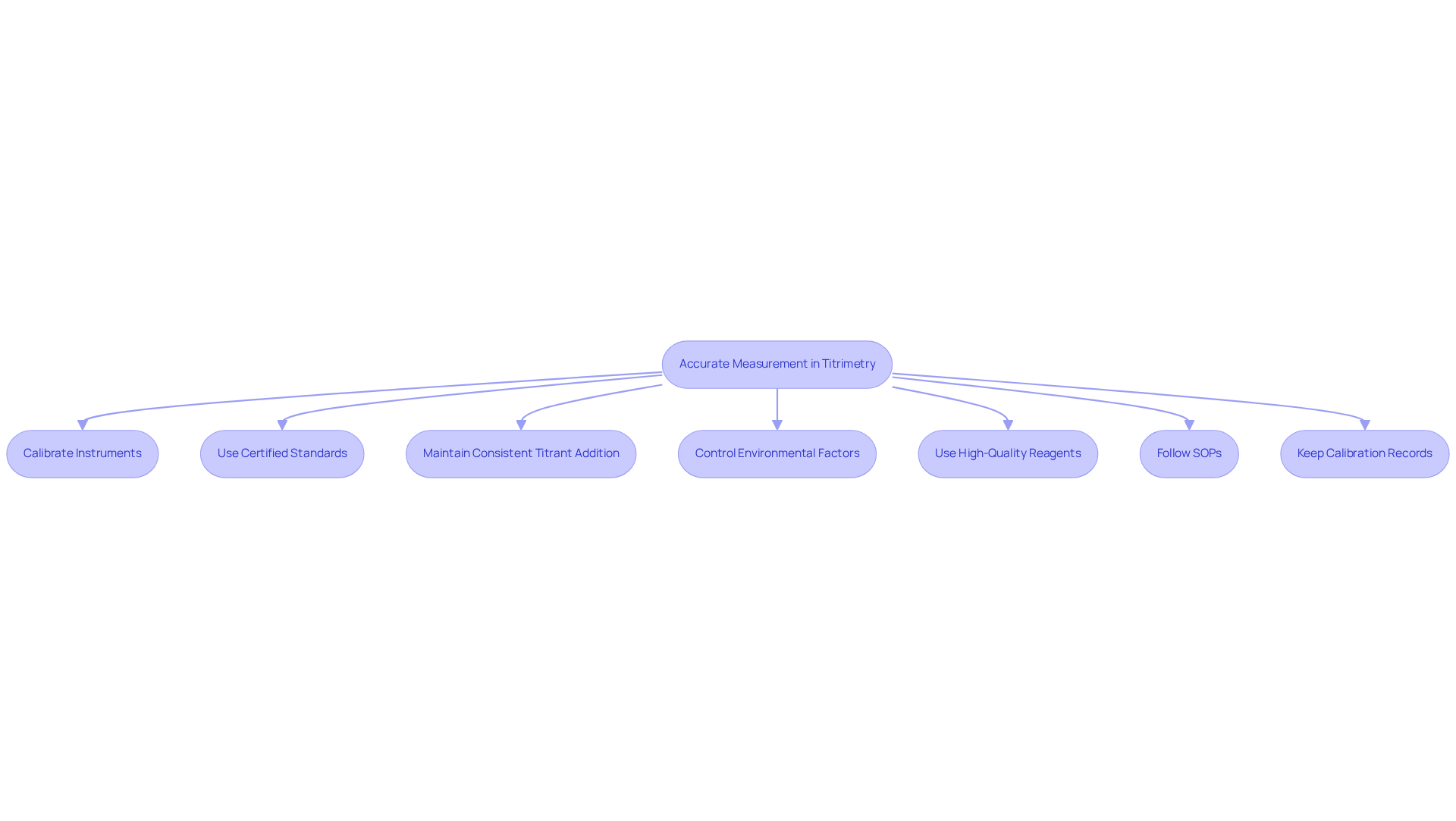
To achieve accurate measurements in titrimetry, it is essential to regularly calibrate all measuring instruments such as pipettes and burettes. Calibration must be performed using certified standards to ensure traceability and reliability. Furthermore, employing proper techniques, such as maintaining a consistent and slow titrant addition rate, significantly minimizes errors. Conducting measurements in a controlled environment is also recommended to reduce the influence of external factors, such as temperature variations and air movements, which can adversely affect outcomes.
Utilizing high-quality reagents and ensuring proper storage conditions will further . Developing and adhering to Standard Operating Procedures (SOPs) for calibration and usage is critical to maintaining systematic approaches. Moreover, keeping comprehensive calibration records is essential for audits and compliance, particularly in pharmaceutical laboratories. It is vital to use only compatible liquids with known properties for calibration solutions to bolster reliability.
By implementing these methods and recognizing common pitfalls, such as using incorrect calibration solutions or neglecting environmental factors, facilities can enhance the dependability of results and improve overall operational efficiency with titrimetry.
Choose Suitable Titration Methods for Applications
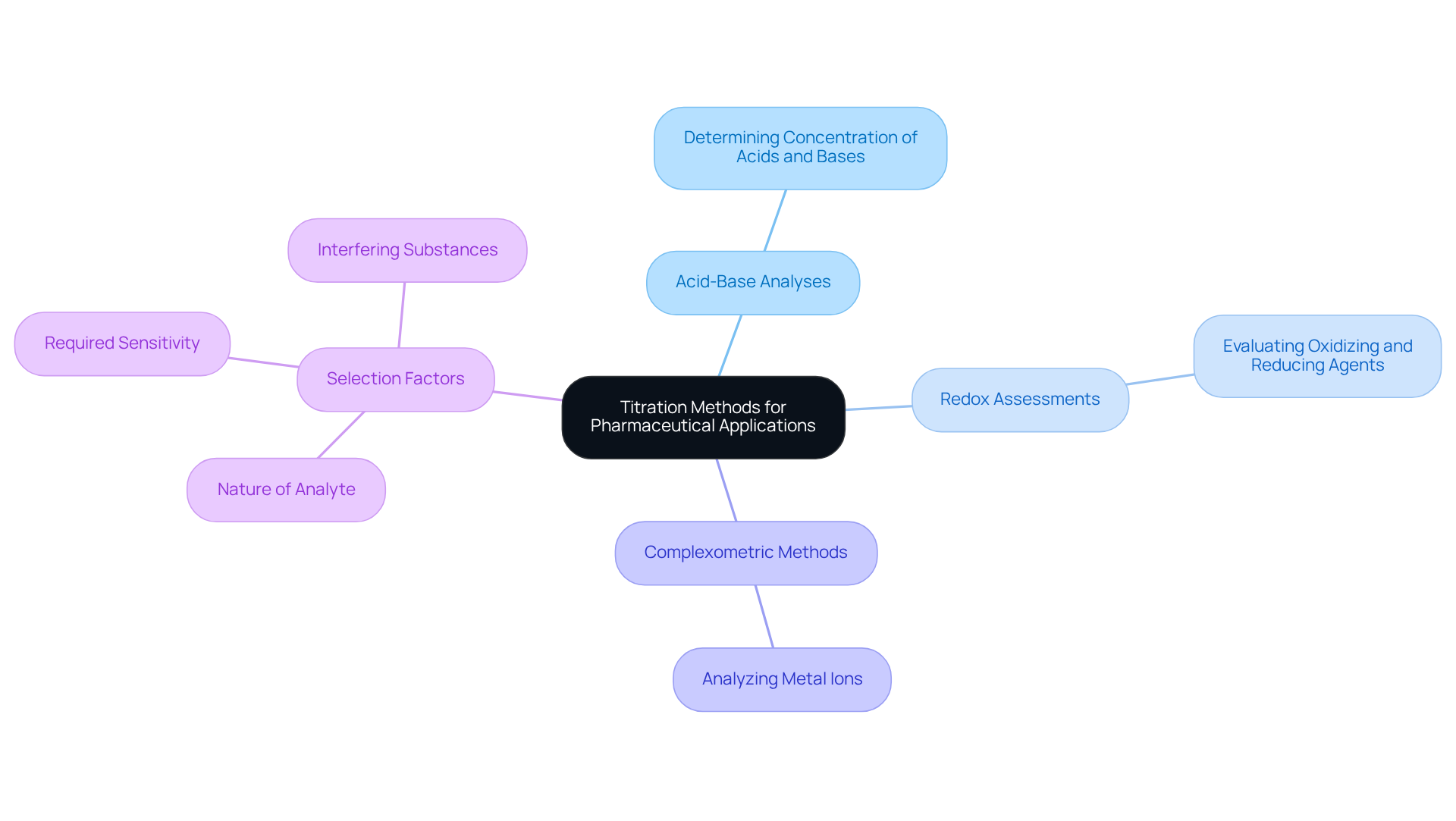
Pharmaceutical laboratories frequently assess a diverse range of substances, making the selection of the appropriate measurement method critical. Acid-base analyses are widely employed to determine the concentration of acids and bases, whereas redox assessments are specifically designed for evaluating oxidizing and reducing agents. Complexometric methods are particularly effective in analyzing metal ions. In selecting the most appropriate method, it is vital to consider factors such as the nature of the analyte, the required sensitivity, and potential interfering substances.
Automated dispensing systems, such as those offered by JM Science Inc., significantly enhance accuracy and minimize human error, proving to be a valuable investment for high-throughput facilities. These systems streamline the measurement process, facilitating consistent and repeatable results. JM Science provides a range of , Karl Fischer reagents, and HPLC solutions that can be seamlessly integrated into laboratory workflows, ensuring optimal performance and precision in outcomes. By carefully aligning the chosen titrimetry technique with specific analytical requirements, facilities can effectively fulfill their essential roles in pharmaceutical development and quality assurance.
Ensure Quality Control and Regulatory Compliance
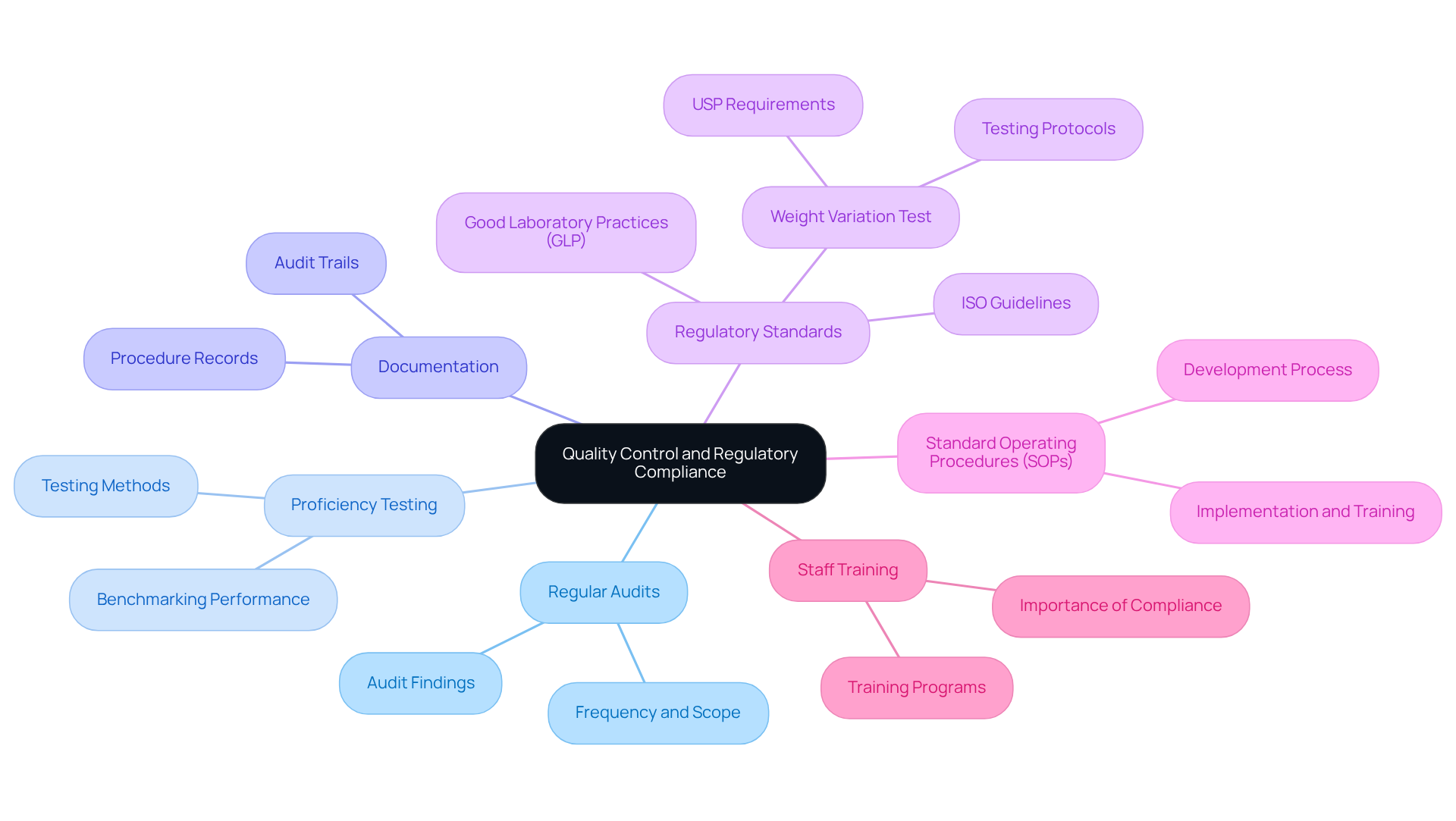
To maintain quality control in titrimetry, it is essential for facilities to implement a comprehensive quality management system that encompasses:
- Regular audits
- Proficiency testing
- Meticulous documentation of all procedures
Following regulatory standards like Good Laboratory Practices (GLP) and ISO guidelines is essential for guaranteeing that laboratory findings are credible and acknowledged by regulatory authorities. For instance, the weight variation test is a non-destructive method used to evaluate the uniformity of pharmaceutical dosage forms, which is crucial for maintaining quality control.
Furthermore, developing standard operating procedures (SOPs) for titrimetry is essential for maintaining consistency and reliability in results. Training staff on these SOPs, while highlighting the importance of , fosters a culture of compliance and excellence within the facility. As stated by USP, "not less than 30 dosage forms should be selected for the weight variation test," underscoring the importance of rigorous testing protocols.
By prioritizing quality control and regulatory adherence, pharmaceutical labs not only enhance their reputation but also contribute significantly to the safety and efficacy of healthcare products. Recent updates, such as the FDA's revised guidance on nitrosamines, further underscore the need for laboratories to stay informed and compliant with evolving regulations.
Conclusion
Mastering titrimetry is essential for pharmaceutical laboratories striving for precision and compliance in their analytical processes. By understanding the fundamentals of titrimetry, implementing accurate measurement techniques, selecting suitable methods for specific applications, and ensuring robust quality control, labs can significantly enhance their operational effectiveness and uphold the highest standards in pharmaceutical analysis.
Key insights from this article highlight the importance of thorough calibration, the careful choice of titration methods based on analyte characteristics, and adherence to regulatory requirements. Regular audits and staff training on standard operating procedures are vital components that foster a culture of excellence and compliance, ultimately contributing to the reliability of laboratory results.
The significance of titrimetry in pharmaceutical analysis cannot be overstated. As regulations evolve, it becomes imperative for laboratories to stay informed and adapt to current trends and practices. Emphasizing best practices in titrimetry not only bolsters the credibility of laboratory findings but also plays a crucial role in ensuring the safety and efficacy of healthcare products. By committing to these practices, pharmaceutical labs can continue to thrive in an ever-changing landscape, delivering accurate and reliable results that support public health.




