Overview
The article delves into the structure and significance of 1,1-dimethylcyclohexane, underscoring its unique conformation and its pivotal role in organic chemistry. This compound's distinctive arrangement of methyl groups not only influences its stability but also its reactivity. As such, it serves as a valuable model for studying steric effects, making it a crucial reagent in both pharmaceutical and materials science applications. Understanding these characteristics is essential for researchers aiming to leverage its properties in various scientific endeavors.
Introduction
In the realm of organic chemistry, few compounds capture the attention of researchers quite like 1,1-dimethylcyclohexane. This cycloalkane, with its intriguing molecular structure, plays a significant role in advancing our understanding of various scientific fields.
As a model compound for studying steric effects and conformational dynamics, 1,1-dimethylcyclohexane provides invaluable insights into the stability and reactivity of cycloalkanes. Its applications span pharmaceuticals and organic synthesis, underscoring its versatility and importance in ongoing research and innovation.
As the scientific community continues to explore its properties, the relevance of 1,1-dimethylcyclohexane grows, promising exciting developments in both academic and industrial settings.
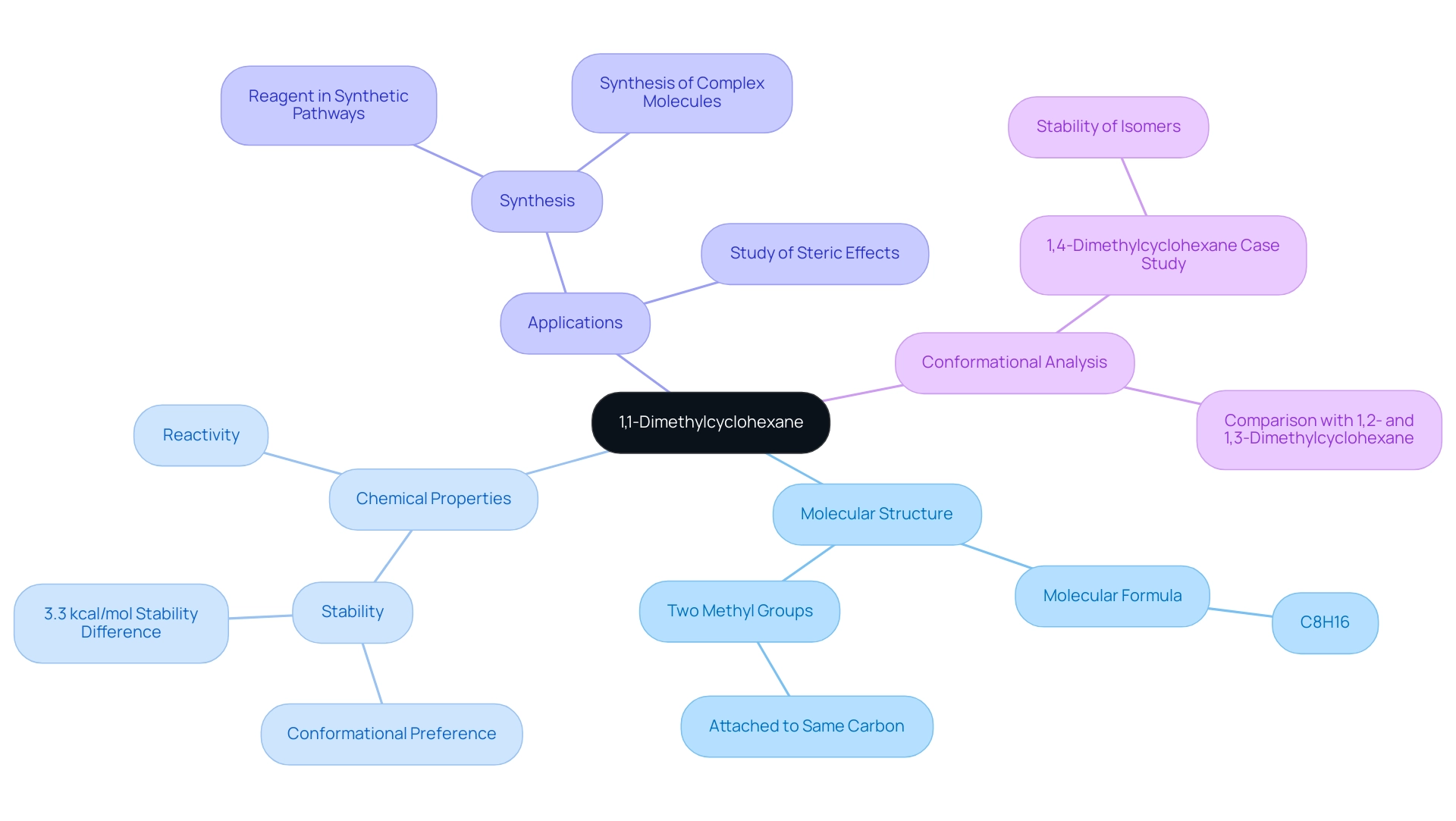
Overview of 1,1-Dimethylcyclohexane: Structure and Importance
1,1-dimethylcyclohexane, with the molecular formula C8H16, stands out as due to its unique structure characterized by two methyl groups attached to the same carbon atom within a cyclohexane ring. This distinctive arrangement significantly influences its chemical properties and stability, making it an essential component in organic chemistry. The compound's importance is underscored by its diverse applications in synthesis and its role as a model substance for investigating steric effects in cycloalkanes.
The resilience of 1,1-dimethylcyclohexane is particularly significant; this compound displays a conformational preference that is vital for comprehending its reactivity and interactions. For example, the conformer with the tert-butyl group in an axial position is approximately 3.3 kcal/mol less stable than its equatorial counterpart, emphasizing the critical role of conformational analysis in predicting the behavior of similar compounds. As Kelly Matthews, Senior Professor of Chemistry, states, "You should find that the isomer of 1,4-dimethylcyclohexane is more stable than the isomer," reinforcing the importance of stability in cycloalkane studies.
In laboratory contexts, 1,1-dimethylcyclohexane functions as a valuable reagent in various synthetic pathways, facilitating the synthesis of more complex molecules. Its applications extend to the examination of reaction mechanisms and the investigation of steric hindrance, both of which are crucial for advancing chemical research. Recent studies have demonstrated its utility in synthesizing derivatives that play pivotal roles in pharmaceuticals and materials science.
Case studies, such as the conformational analysis of 1,4-dimethylcyclohexane, further illustrate the compound's significance. These analyses indicate that the isomer of 1,4-dimethylcyclohexane is expected to be more stable than its counterparts, mirroring findings from studies on 1,2- and 1,3-dimethylcyclohexane. Such insights not only enhance our understanding of cycloalkane behavior but also inform the development of new substances in organic synthesis.
JM Science Inc. consistently updates its product offerings and fosters strong relationships with manufacturers to address the evolving needs of the scientific community. The ongoing exploration of specific compounds in scientific research continues to yield valuable insights, solidifying its status as a cornerstone in organic chemistry. As researchers delve deeper into its properties and applications, the substance remains a focal point for studies aimed at unraveling the complexities of cycloalkanes and their derivatives.
Chemical Structure of 1,1-Dimethylcyclohexane: An In-Depth Analysis
The chemical structure of this compound prominently features a cyclohexane ring with two methyl groups (−CH₃) attached to the first carbon atom, resulting in the molecular formula C8H16. This configuration indicates the presence of eight carbon atoms and sixteen hydrogen atoms. The unique positioning of the two methyl groups on the same carbon introduces a distinct steric environment that significantly impacts the compound's stability and reactivity.
Visually, this structure can be represented as follows:
CH3
|\
CH3-C-CH2
| |
CH2-CH2-CH2
The spatial arrangement of the atoms is crucial for understanding the conformational behavior of 1,1-dimethylcyclohexane.
Research indicates that the introduction of methyl groups can enhance the robustness of cyclohexane derivatives by reducing torsional strain and steric hindrance. For instance, studies have demonstrated that the presence of these groups can lead to a more favorable chair conformation, which is recognized as the most stable form of cyclohexane. Notably, the closed cup flash point of the substance is 11 °C, a critical factor for its safety and management in laboratory environments.
Furthermore, the steric effects associated with the methyl groups can influence the reactivity of the material in various reactions. Current research underscores that these effects play an essential role in determining the outcomes of reactions involving cycloalkanes, rendering this compound a significant subject of study in organic chemistry. The implications of these findings are particularly relevant for applications in pharmaceuticals and materials science, where understanding the stability and reactivity of substances is paramount.
Additionally, a specific additive has been recognized for facilitating mild-pressure handling of natural-gas hydrate, highlighting its contemporary relevance in ongoing research and industry practices.
In the context of , it is imperative to acknowledge the copyright of data aggregation by the U.S. Secretary of Commerce on behalf of the U.S.A., as stated by William E. Wallace, director at NIST Mass Spectrometry Data Center. This emphasizes the importance of reliable data in the examination of chemical compounds such as certain cyclic hydrocarbons. Moreover, JM Science Inc.'s commitment to quality and support in providing high-performance analytical instruments further distinguishes the company in the market, particularly concerning products and research relevant to 1,1-dimethylcyclohexane.
Conformations of 1,1-Dimethylcyclohexane: Chair and Boat Forms
1,1-Dimethylcyclohexane exhibits multiple conformations, with the chair and boat forms being the most prominent. The chair conformation is recognized as the most stable due to its ability to minimize both steric and torsional strain. In this arrangement, the two methyl groups can occupy equatorial positions, significantly reducing steric hindrance and improving overall balance.
In contrast, the boat conformation is inherently less stable, primarily due to increased steric interactions between the methyl groups, which can lead to higher energy states. Recent studies have highlighted the importance of conformational analysis in organic compounds, particularly in understanding their reactivity and interactions during chemical reactions. For instance, the reliability of the chair conformation over the boat conformation is not merely theoretical; it has been supported by computational analyses that demonstrate the chair form's lower energy state. Specifically, dehydrogenation of cyclohexane begins to show noticeable changes at temperatures exceeding 300 °C, indicating the relevance of these conformations in practical applications.
Furthermore, a comparison of low energy and experimental structures has reinforced the understanding of these conformations. Moreover, a case study titled "Neighbor-list Reduction: Optimization for Computation of Molecular Van der Waals and " introduced a method that significantly improved computational efficiency in analyzing molecular surface areas. This optimization achieved substantial reductions in processing time while maintaining accuracy, underscoring the practical implications of conformational stability in computational chemistry.
As Jasna J. Klicić noted, "We have developed a protocol for computing the acidity constant (pKa) of organic substances via ab initio quantum chemistry and continuum solvation methods," which emphasizes the relevance of computational techniques in understanding molecular behavior. In summary, understanding the chair and boat conformations of 1,1-dimethylcyclohexane is essential for predicting the substance's behavior in various scientific contexts. The preference for the chair conformation, especially when larger substituents are present, is a key factor in minimizing steric hindrance and enhancing durability, making it a fundamental concept in the study of cycloalkanes.
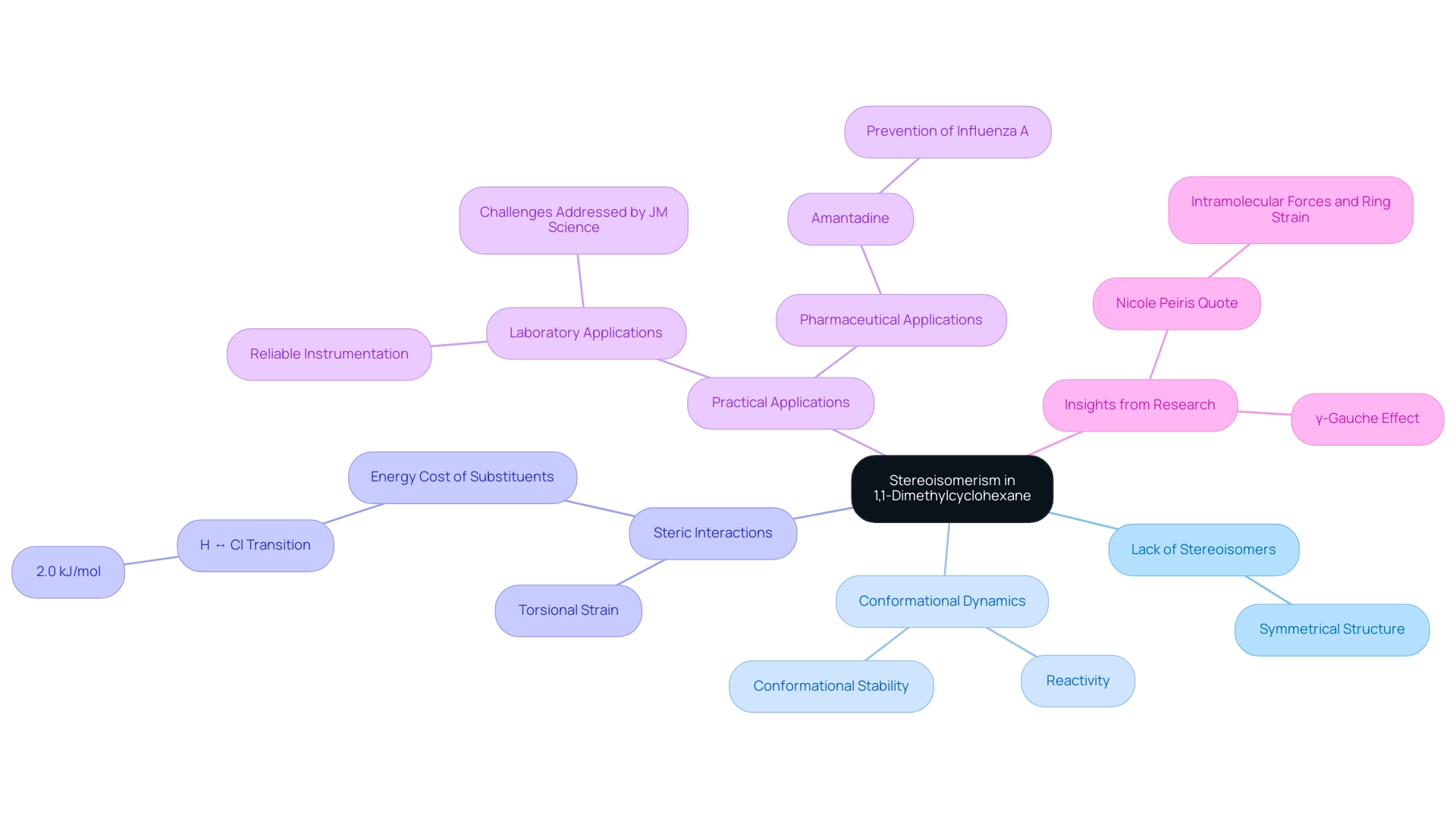
Stereoisomerism in 1,1-Dimethylcyclohexane: Understanding Isomers
The study of cycloalkanes presents a unique case with 1,1-dimethylcyclohexane, which does not exhibit stereoisomerism in the conventional sense. This phenomenon arises from both methyl groups being bonded to the same carbon atom, resulting in a symmetrical structure that lacks distinct stereoisomers. However, the compound's ability to adopt various conformations plays a crucial role in its reactivity and overall behavior.
The structural integrity of this compound is influenced by factors such as steric interactions and torsional strain, which can significantly impact its reactivity. For instance, the energy cost associated with substituent changes, such as the transition from hydrogen to chlorine, is quantified at 2.0 kJ/mol, illustrating the subtle energy dynamics at play.
Studies suggest that although this compound may lack stereoisomers, the conformational changes it experiences can lead to varying reactivities. This emphasizes the significance of understanding conformational stability, as it directly influences the compound's interactions and reactions in diverse environmental contexts.
Moreover, the examination of stereoisomerism in cycloalkanes has uncovered insights into the γ-gauche effect, particularly in 1,1-dimethylcyclohexane, where configurations can affect shifts in properties. As noted by Nicole Peiris, "they play a role in cycloalkanes because they refer to that cause ring strain." This phenomenon underscores the relevance of conformational analysis in predicting substance behavior, making it a vital area of focus in contemporary research.
In practical applications, understanding these dynamics is crucial for laboratories, particularly in the context of JM Science's commitment to providing reliable instrumentation for precise measurements. The insights gained from conformational resilience can enhance the efficacy of chemical analyses, thereby addressing challenges faced by laboratories in obtaining accurate results.
Additionally, the importance of conformational integrity extends to pharmaceutical applications, as observed with compounds like amantadine, which is effective in preventing influenza A infections. This connection highlights the broader implications of conformational reliability in drug design and efficacy.
In summary, although 1,1-dimethylcyclohexane may lack traditional stereoisomers, its conformational dynamics are essential for understanding the reactivity and interactions of the compound, reinforcing the importance of conformational integrity in the broader context of cycloalkane chemistry.
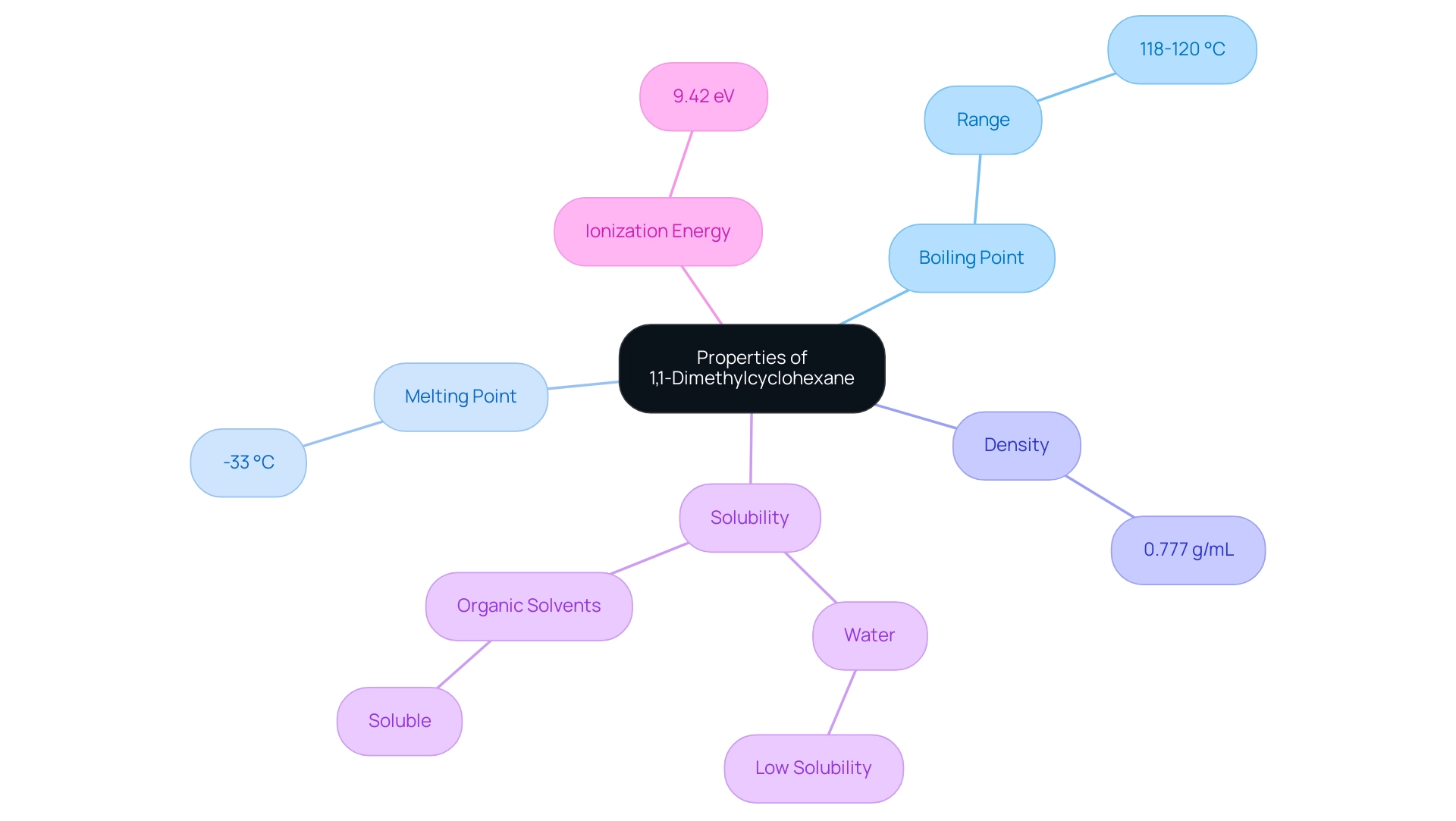
Physical and Chemical Properties of 1,1-Dimethylcyclohexane
The physical and structural properties of 1,1-dimethylcyclohexane are significant and essential for its application in laboratory environments. This compound exhibits a boiling point ranging from approximately 118 to 120 °C and a melting point of -33 °C, indicating its stability under . Its relatively non-polar nature results in low solubility in water; however, it remains soluble in organic solvents, making it a versatile choice for numerous applications.
The density of 1,1-dimethylcyclohexane is approximately 0.777 g/mL, a critical factor to consider when utilizing it as a solvent in organic synthesis. Additionally, the ionization energy of 1,1-dimethylcyclohexane is determined to be 9.42 eV, as reported by Sieck and Mautner (Meot-Ner, 1982), further emphasizing its properties. These characteristics not only facilitate its role as a reagent in various reactions but also highlight its suitability for applications requiring precise measurements and reliable performance.
Understanding these properties is essential for laboratory experts, particularly in pharmaceutical environments, where the precise and efficient application of substances is vital.
Recent research in 2025 has further investigated the boiling and melting points of cycloalkanes, providing updated insights into their physical characteristics, particularly those of 1,1-dimethylcyclohexane. This advancement enhances the knowledge base for effective laboratory practices. Furthermore, JM Science Inc.'s commitment to quality and customer support ensures that laboratory professionals have access to reliable instrumentation and resources, which is crucial when working with substances such as 1,1-dimethylcyclohexane. For additional information on data and usage terms, institutions may already be subscribers to NIST subscription sites, offering valuable resources.
Applications of 1,1-Dimethylcyclohexane in Industry and Research
The compound 1,1-Dimethylcyclohexane plays a pivotal role in various applications, particularly within organic synthesis and materials science. As a solvent, it promotes a range of reactions and serves as a reagent in the creation of more intricate organic substances. Its unique structural properties render it an invaluable model substance for studying steric effects and conformational analysis in cycloalkanes, providing insights that are crucial for advancing scientific understanding.
In the pharmaceutical industry, 1,1-Dimethylcyclohexane is increasingly recognized for its utility in drug development and formulation processes. Recent advancements in organic synthesis have underscored its effectiveness in creating novel substances, essential for developing new therapeutic agents. While the octane number for 1,1-Dimethylcyclohexane is not explicitly stated, it is noteworthy that the octane number of 1-Methyl-2-n-propylcyclohexane measures at 441.10, highlighting the potential of cyclohexane derivatives, including 1,1-Dimethylcyclohexane, as high-performance solvents in various applications. Expert insights from pharmaceutical researchers emphasize the significance of 1,1-Dimethylcyclohexane in enhancing the efficiency of drug development processes.
Its ability to dissolve a wide range of substances makes it a preferred choice for formulating drugs that require precise solubility profiles. Furthermore, case studies on industrial applications demonstrate how 1,1-Dimethylcyclohexane has been successfully employed in , utilizing Multidimensional Scaling (MDS) software to visualize and filter solvent data based on physical properties. This capability allows researchers to tailor solvent choices to specific applications, thereby optimizing decision-making in solvent selection. As noted by Byrne et al., relying solely on the existing catalogue of largely conventional solvents limits the availability of green solvent substitutes for various applications.
This underscores the importance of investigating options such as 1,1-Dimethylcyclohexane in the pursuit of more sustainable solutions in industrial processes. Overall, the current uses of 1,1-Dimethylcyclohexane in pharmaceuticals and its role in organic synthesis highlight its significance in research and development, making it a substance of interest for ongoing studies and innovations in the field.

Key Takeaways: The Significance of 1,1-Dimethylcyclohexane in Chemistry
The substance 1,1-dimethylcyclohexane is pivotal in organic chemistry, distinguished by its unique structure and conformational dynamics. This compound, with a ChemSpider ID of 11062, serves as a critical model for understanding steric effects, essential in various chemical reactions and interactions. Its conformational behavior, characterized by the presence of axial and equatorial positions, significantly influences its reactivity and stability, making it a valuable subject of study in both academic and industrial settings.
The importance of 1,1-dimethylcyclohexane extends into the pharmaceutical realm, where its derivatives are explored for potential therapeutic applications. Research indicates that cycloalkanes, including certain derivatives, contribute to the development of novel drug formulations, enhancing bioavailability and efficacy. Ongoing research emphasizes the influence of cycloalkanes on pharmaceutical development, particularly their effects on molecular interactions and pharmacokinetics.
In 2025, the significance of a specific cycloalkane in modern chemistry is further highlighted by its role in innovative research and development initiatives. Case studies illustrate how this substance assists in creating intricate molecules, underscoring its flexibility and importance in modern scientific inquiry. JM Science Inc. plays an essential role in this environment by offering high-quality products such as premium titrators, , HPLC columns, and innovative medical devices that aid researchers in their investigations.
Their extensive support resources, including how-to videos and application libraries, are crucial for researchers, ensuring access to reliable and efficient instrumentation that facilitates advancements in pharmaceutical research. Additionally, recent data on the constant pressure heat capacity of gas at various temperatures—ranging from 38.15 J/molK at 50 K to 548.7 J/molK at 3000 K—further contextualizes the thermodynamic properties of 1,1-dimethylcyclohexane and its applications in chemical research. JM Science Inc. offers competitive pricing on HPLC fittings, manual injection valves, and HPLC solvent reservoir kits, ensuring laboratories have access to the necessary tools for their analyses.
The dedication of companies like JM Science Inc. to innovation and maintaining strong relationships with top manufacturers ensures that researchers possess the tools necessary to explore the full potential of 1,1-dimethylcyclohexane and other compounds.
Conclusion
The exploration of 1,1-dimethylcyclohexane underscores its critical role in organic chemistry, particularly as a model compound that enhances our understanding of steric effects and conformational dynamics. Its unique structure, defined by two methyl groups on the same carbon atom, not only influences its stability and reactivity but also establishes it as an invaluable asset in various applications, including organic synthesis and pharmaceuticals. The compound's ability to adopt multiple conformations, particularly the more stable chair form, emphasizes its significance in predicting chemical behavior and interactions.
The implications of 1,1-dimethylcyclohexane extend well beyond theoretical studies, reaching into practical applications in drug development and formulation processes. By facilitating the synthesis of complex molecules and enhancing the solubility profiles of therapeutic agents, this compound plays a pivotal role in advancing pharmaceutical research. Moreover, its properties have inspired innovative approaches in solvent selection, thereby promoting the pursuit of greener alternatives in chemical processes.
As research continues to unveil the complexities of 1,1-dimethylcyclohexane, its relevance in both academic and industrial settings is increasingly acknowledged. The ongoing commitment of organizations like JM Science Inc. to provide high-quality analytical instruments and support resources ensures that researchers are equipped with the necessary tools to explore the full potential of this compound. In summary, 1,1-dimethylcyclohexane stands at the forefront of organic chemistry, promising exciting developments and innovations that will shape the future of the field.




