Overview
This article delves into polarography, a pivotal voltammetric method essential for measuring current flow in relation to applied voltage. Primarily utilizing a dropping mercury electrode (DME), polarography enables precise analysis of electroactive species. Its versatility spans various fields, notably pharmaceuticals and environmental monitoring, underscoring its historical significance. Furthermore, advancements in polarography have markedly enhanced its sensitivity and applicability within modern analytical chemistry. This comprehensive examination not only highlights the method's importance but also reinforces its relevance in contemporary scientific practices.
Introduction
Polarography serves as a cornerstone of analytical chemistry, recognized for its ability to elucidate the complexities of electroactive species through current measurements in response to applied voltage. This versatile technique, particularly distinguished by its dropping mercury electrode, significantly enhances the precision of both qualitative and quantitative analyses. Its crucial applications span various fields, including pharmaceuticals and environmental monitoring. As the demand for accuracy and safety in chemical analysis intensifies, it prompts a critical examination:
- How has polarography adapted to meet these evolving challenges?
- What renders it indispensable in contemporary research and industry?
Define Polarography: Principles and Techniques
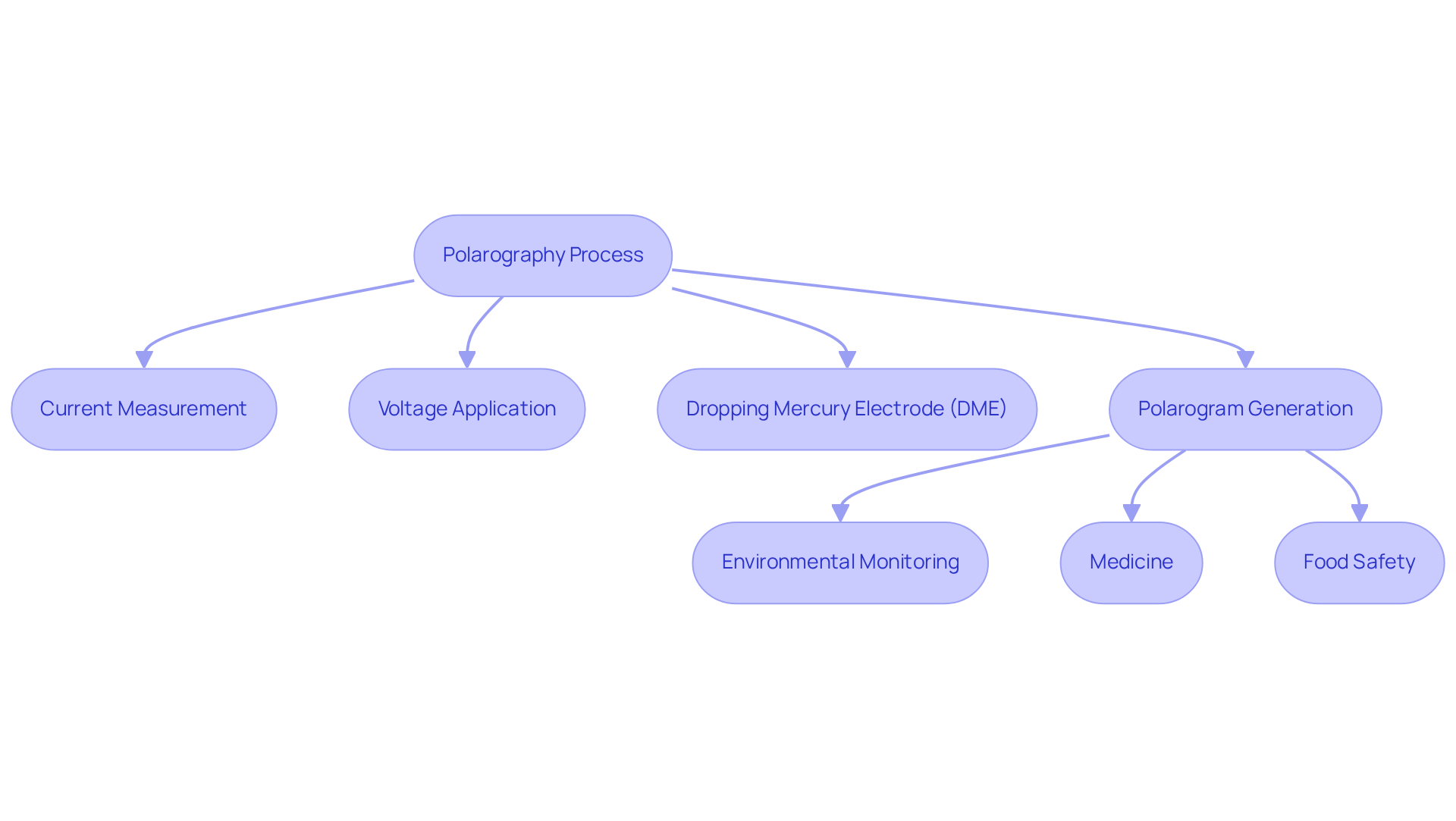
Polarography is recognized as a pivotal voltammetric method, defined by the measurement of current flow through a solution in relation to an applied voltage. Central to this technique is the dropping mercury electrode (DME), which provides a fresh surface for each measurement, enhancing accuracy and reliability. This method is indispensable for analyzing electroactive species in solution, offering both qualitative and quantitative insights. The current response is systematically plotted against the applied potential, resulting in a polarogram that elucidates crucial information regarding the concentration and identity of the analytes involved in the polarography.
The versatility of polarography encompasses various domains, such as environmental monitoring, medicine, and food safety. Its ability to deliver precise data makes it an invaluable tool for researchers and professionals alike. By employing this technique, one can not only identify the presence of specific substances but also quantify their concentrations, thereby facilitating informed decision-making in critical applications. The implications of polarography are profound, as it enhances our understanding of chemical interactions in diverse environments.
In summary, polarography serves as a cornerstone of modern analytical chemistry, empowering scientists to explore the complexities of electroactive species, rather than being merely a method. As the demand for high-quality scientific instruments continues to grow, it becomes essential to embrace polarography in laboratory settings to advance research and ensure safety across various sectors.
Contextualize Polarography in Pharmaceutical Analysis
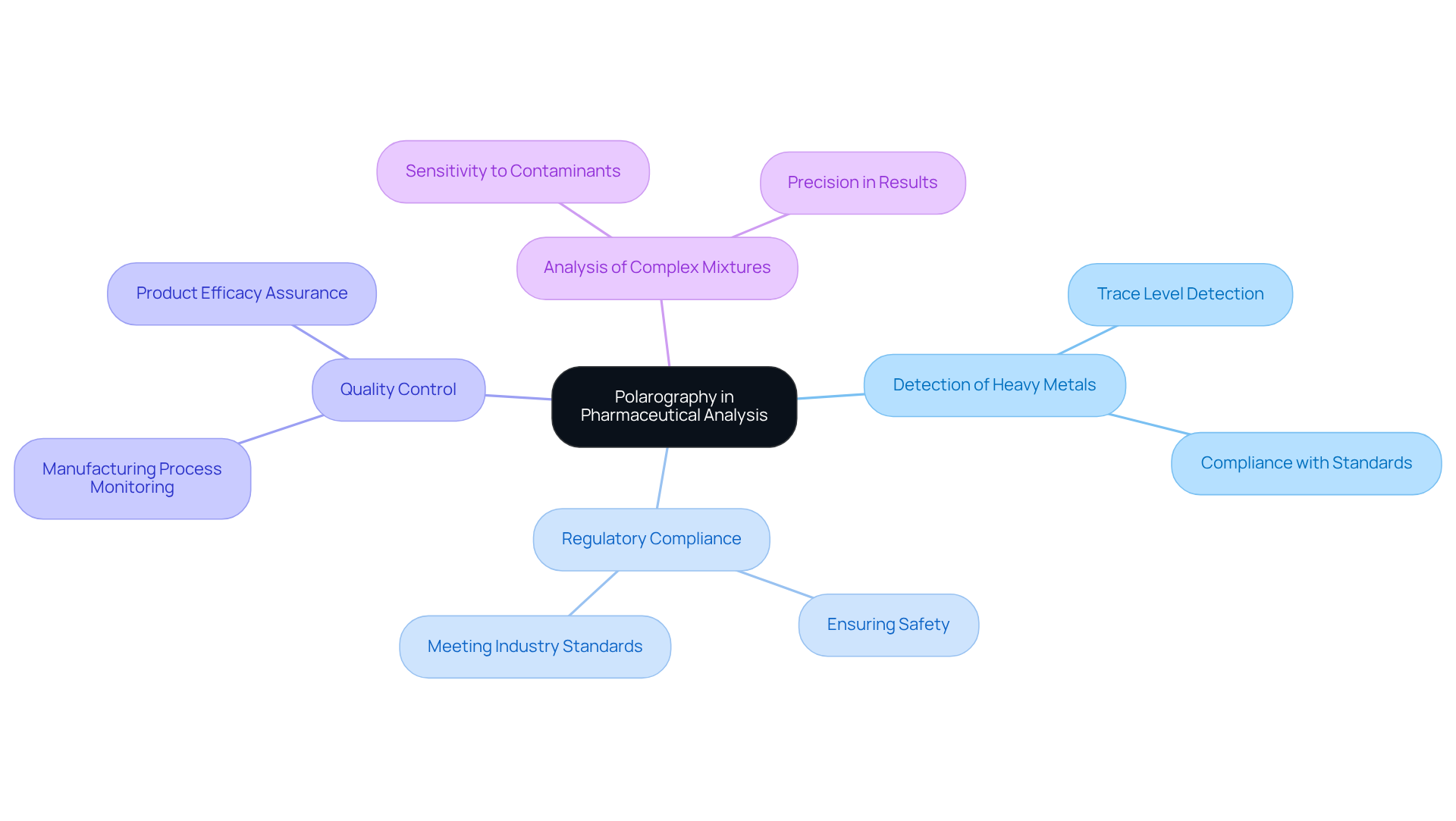
In the drug industry, a specific electrochemical technique plays a pivotal role in examining active ingredients and their degradation products. This technique is particularly advantageous for detecting trace levels of metals and other contaminants in drug formulations.
For instance, employing this electrochemical method allows for the precise ascertainment of heavy metal levels in medicinal products, ensuring compliance with regulatory standards.
Furthermore, the capability of polarography to analyze complex mixtures establishes it as a valuable tool for quality control during the manufacturing process. Its sensitivity and precision significantly contribute to the overall safety and efficacy of pharmaceutical products, solidifying its status as an essential method in the field.
Trace the Origins and Evolution of Polarography
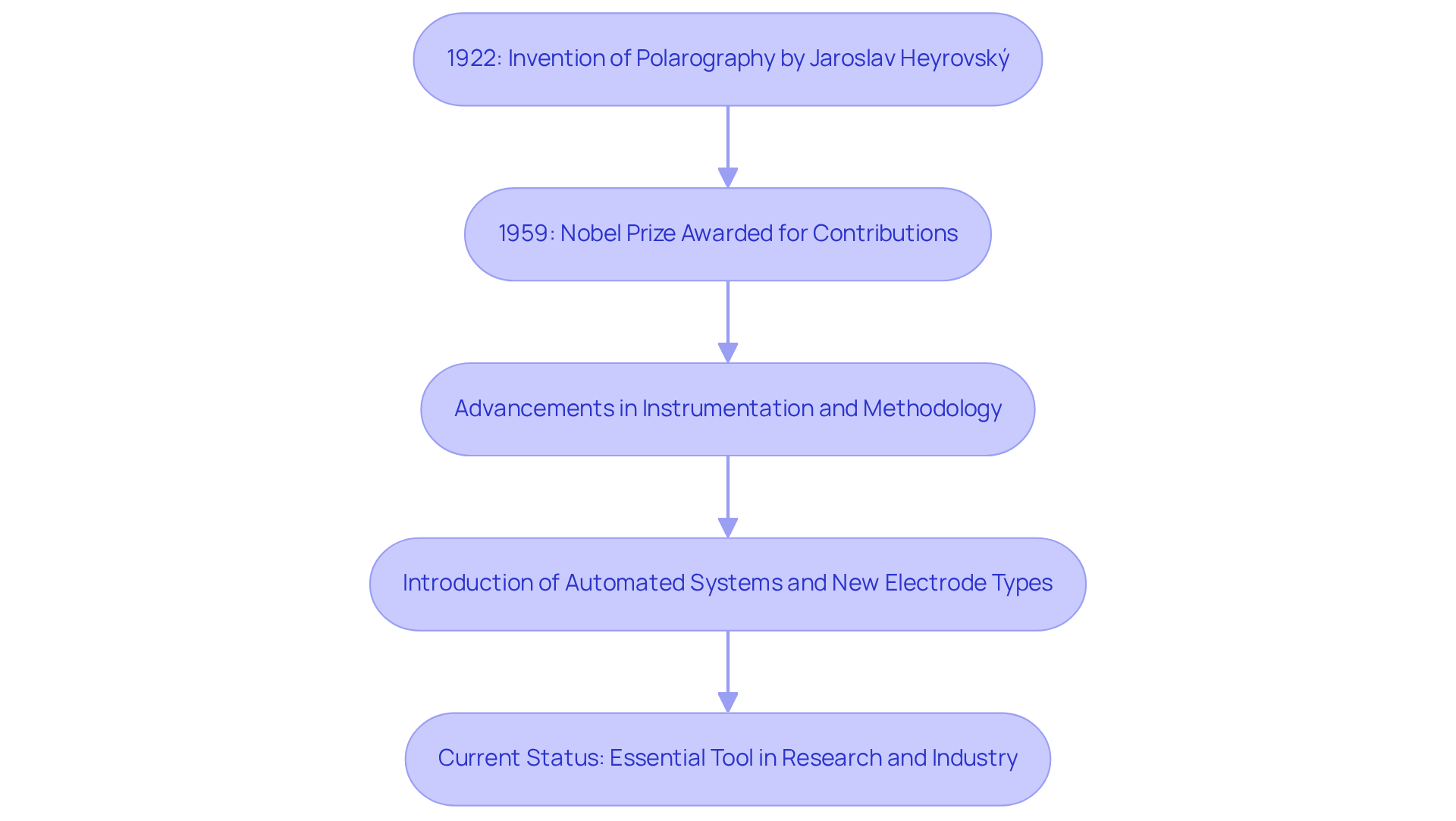
The analytical technique known as polarography was first developed in 1922 by Czech chemist Jaroslav Heyrovský, who introduced the innovative dropping mercury device to study electrochemical processes. His groundbreaking work not only laid the foundation for [electroanalytical chemistry](https://jmscience.com) but also earned him the Nobel Prize in Chemistry in 1959, underscoring the significance of his contributions.
Over the decades, this technique has undergone substantial evolution, with advancements in instrumentation and methodology that have significantly enhanced its sensitivity and applicability. The introduction of automated systems in polarography, along with the development of various electrode types, has expanded the technique's utility across diverse analytical applications, including environmental monitoring and clinical diagnostics.
Today, polarography is recognized as an essential tool in both research and industry, continuously adapting to satisfy the demands of modern analytical chemistry.
Examine Key Characteristics and Methodologies of Polarography
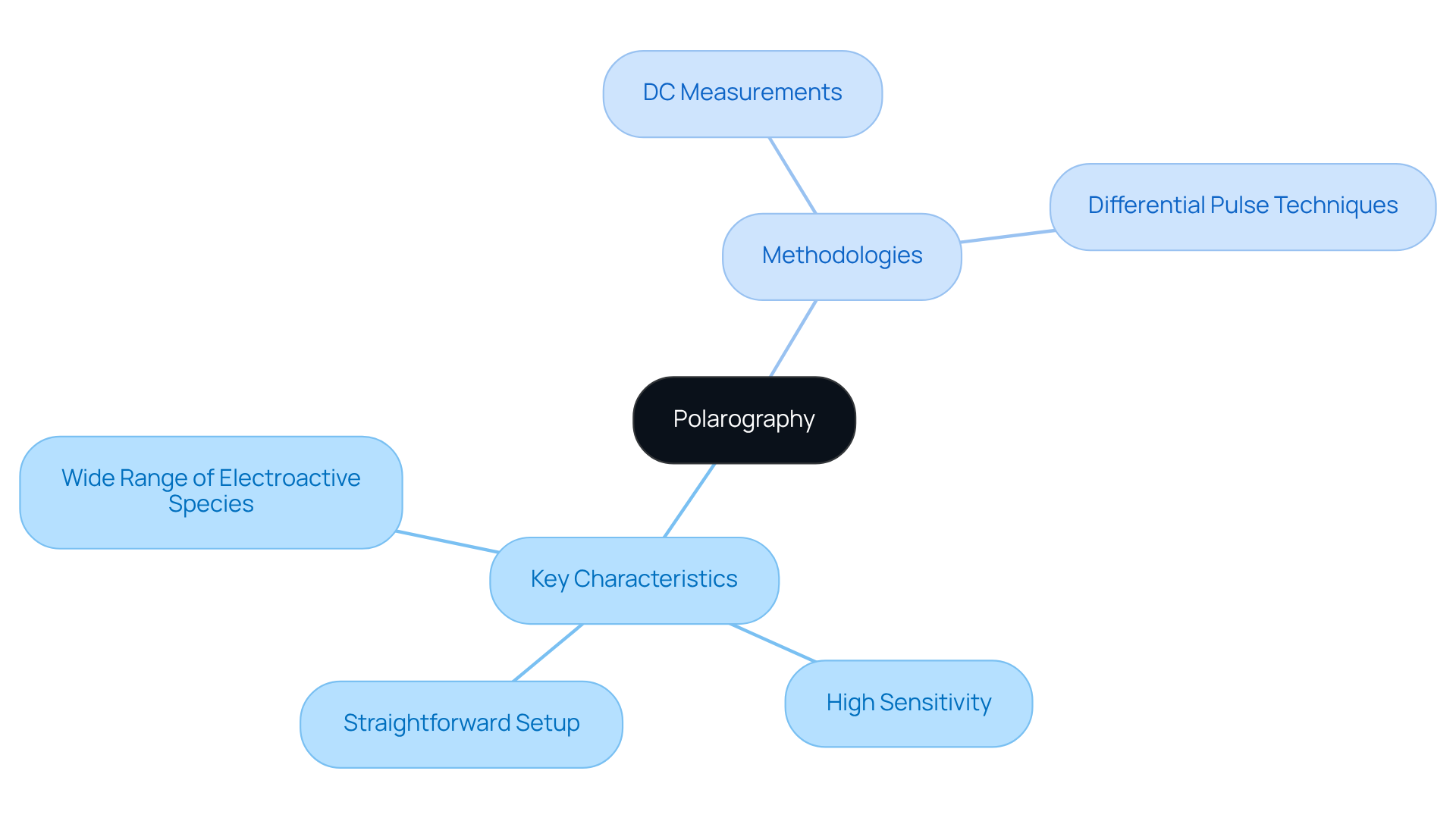
The analytical technique in question is distinguished by several key characteristics:
- its capacity to analyze a wide range of electroactive species,
- its heightened sensitivity to low concentrations,
- its relatively straightforward setup.
Central to this technique is the three-electrode system, which comprises a working electrode (DME), a reference electrode, and a counter electrode. As the voltage is varied, the current response is meticulously measured, facilitating the generation of a polarogram through polarography.
To enhance sensitivity and resolution, various methodologies can be employed, including:
- direct current (DC) measurements
- differential pulse techniques
The selection of a particular methodology is often dictated by the specific requirements of the analysis, such as the nature of the sample and the desired detection limits. Ultimately, the effectiveness and versatility of polarography make it a preferred choice for numerous analytical applications.
Conclusion
Polarography stands as a fundamental technique in analytical chemistry, characterized by its ability to measure current flow in relation to applied voltage. This method, primarily utilizing the dropping mercury electrode, not only enhances measurement accuracy but also serves as a critical tool across diverse fields, from environmental monitoring to pharmaceutical analysis. By providing insights into the concentration and identity of electroactive species, polarography empowers scientists and professionals to make informed decisions based on reliable data.
The discussion has highlighted polarography's versatility and significance. Its origins trace back to the pioneering work of Jaroslav Heyrovský, whose innovations laid the groundwork for modern electroanalytical chemistry. The technique's evolution, marked by advancements in instrumentation and methodologies, has further solidified its relevance in contemporary research and industry. The sensitivity and precision of polarography make it particularly valuable in pharmaceutical applications, where it ensures the safety and efficacy of medicinal products by detecting contaminants and analyzing active ingredients.
The implications of polarography extend beyond mere analysis; they touch upon the broader landscape of scientific inquiry and public safety. As the demand for high-quality analytical methods continues to rise, embracing polarography will be crucial for advancing research and upholding safety standards across various sectors. Engaging with this technique not only enriches understanding of electrochemical processes but also fosters innovation in addressing the challenges of modern science.




