Overview
Alkalinity plays a critical role in measuring the capacity of water to neutralize acids, a factor essential for maintaining stable pH levels across various environments, particularly within laboratories and aquatic ecosystems. It is imperative to understand that adequate alkalinity is vital for ensuring the stability of drug formulations and the accuracy of analytical outcomes. Moreover, it protects aquatic life, underscoring its significance in both pharmaceutical testing and environmental health. This highlights the necessity for professionals in these fields to prioritize alkalinity management, ensuring optimal conditions for both research and ecological preservation.
Introduction
Alkalinity serves as a fundamental pillar in preserving the delicate equilibrium of pH levels in both natural and laboratory settings. As a critical measure of a solution's capacity to neutralize acids, grasping the concept of alkalinity is paramount for ensuring the stability of chemical reactions and safeguarding the health of aquatic ecosystems.
Beyond environmental implications, alkalinity holds significant importance in pharmaceutical and medical laboratories, where meticulous pH management can profoundly influence drug formulation and patient safety.
What challenges emerge in the pursuit of optimal alkalinity levels? Moreover, how do advancing measurement techniques navigate these complexities? These questions underscore the necessity of understanding alkalinity in various scientific domains.
Define Alkalinity: Measurement and Significance
What does alkalinity measure? It is defined as the capacity of water to neutralize acids, a crucial factor in maintaining stable pH levels across various environments. The concentration of bicarbonates, carbonates, and hydroxides present in a solution is what does alkalinity measure. Serving as a buffer, alkalinity helps resist pH changes that can adversely affect aquatic life and chemical processes in laboratories.
In medicinal laboratories, sustaining appropriate pH levels is essential to ensure the stability of drug formulations and the accuracy of analytical outcomes. The Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators are specifically designed for such applications. They offer precise measurement capabilities and comply with Japanese Pharmacopoeia standards, thereby enhancing the reliability of pharmaceutical testing.
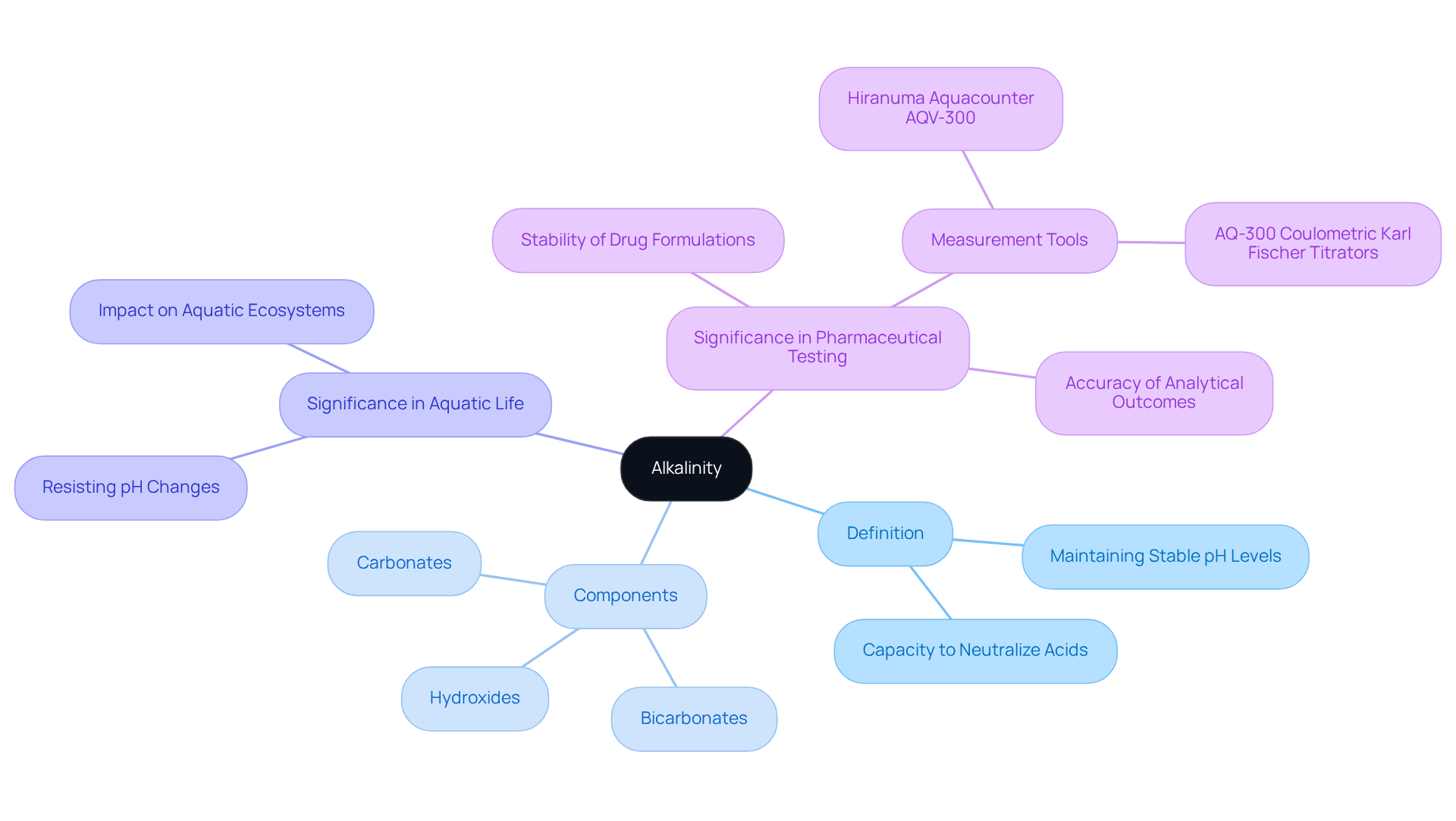
Explore the Relationship Between Alkalinity and pH
Alkalinity and pH are closely related yet distinct concepts that play a critical role in various scientific and environmental contexts. pH measures the concentration of hydrogen ions in a solution, indicating its acidity or basicity, while alkalinity measures the capacity of a solution to neutralize acids. Typically, elevated basicity correlates with a stable pH, which is essential for numerous chemical reactions and biological processes.
For example, in water-based ecosystems, higher pH levels provide buffering against acid rain, thereby protecting aquatic organisms from harmful pH fluctuations. Furthermore, in experimental settings, maintaining a consistent pH through adequate alkalinity is vital for achieving precise measurements and reliable results.
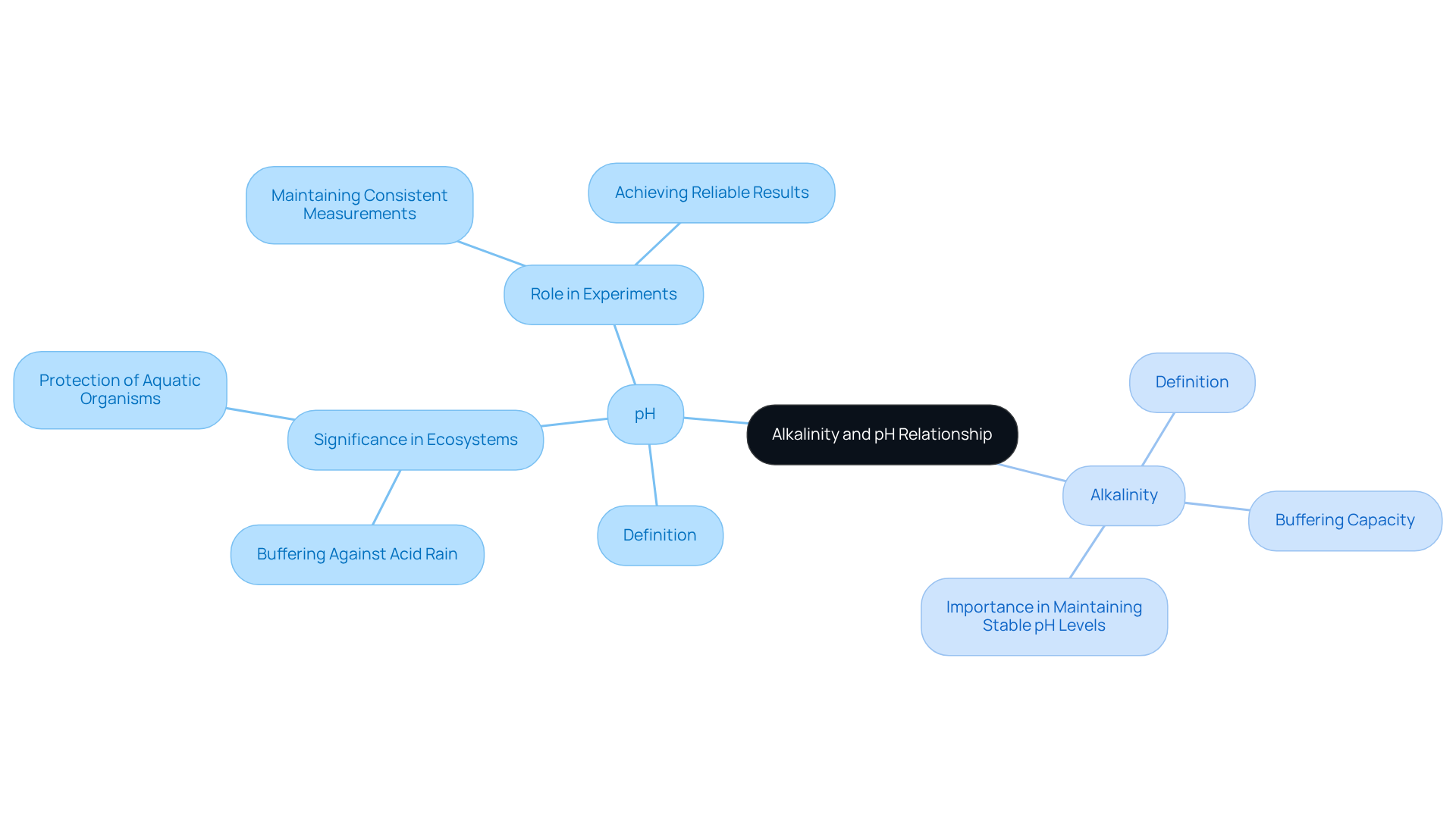
Assess the Role of Alkalinity in Pharmaceutical and Medical Laboratories
In drug and medical laboratories, the role of basicity is paramount for ensuring the stability and effectiveness of drug products. Numerous formulations necessitate specific pH levels to preserve their efficacy, making the observation of basicity essential. For instance, the solubility and bioavailability of active pharmaceutical ingredients can be significantly influenced by basicity; a higher pH can enhance the solubility of certain compounds, thereby improving their absorption in the body.
Furthermore, pH assessments are integral to quality assurance procedures, ensuring that the water utilized in formulations adheres to strict standards. This compliance is crucial for safeguarding patient safety and maintaining product integrity. Regular testing in medical facilities, such as serum alkaline phosphatase, is often conducted for diagnostic and screening purposes, underscoring the importance of precise acidity measurement in clinical environments. The AGA Technical Review on liver chemistry tests provides compelling evidence for assessing liver enzyme outcomes, further highlighting the critical nature of pH balance in preserving product integrity and ensuring patient safety.
Alkalinity also plays a pivotal role in wastewater treatment within medical facilities, where it is employed to neutralize acidic waste prior to discharge, thereby protecting both the environment and public health. Understanding and controlling pH levels is essential for the success of pharmaceutical and medical testing operations, particularly given that for the reference range while remaining normal for the measured population. Ultimately, the effects of basicity extend beyond product formulation to encompass broader management aspects in research facilities and patient care.
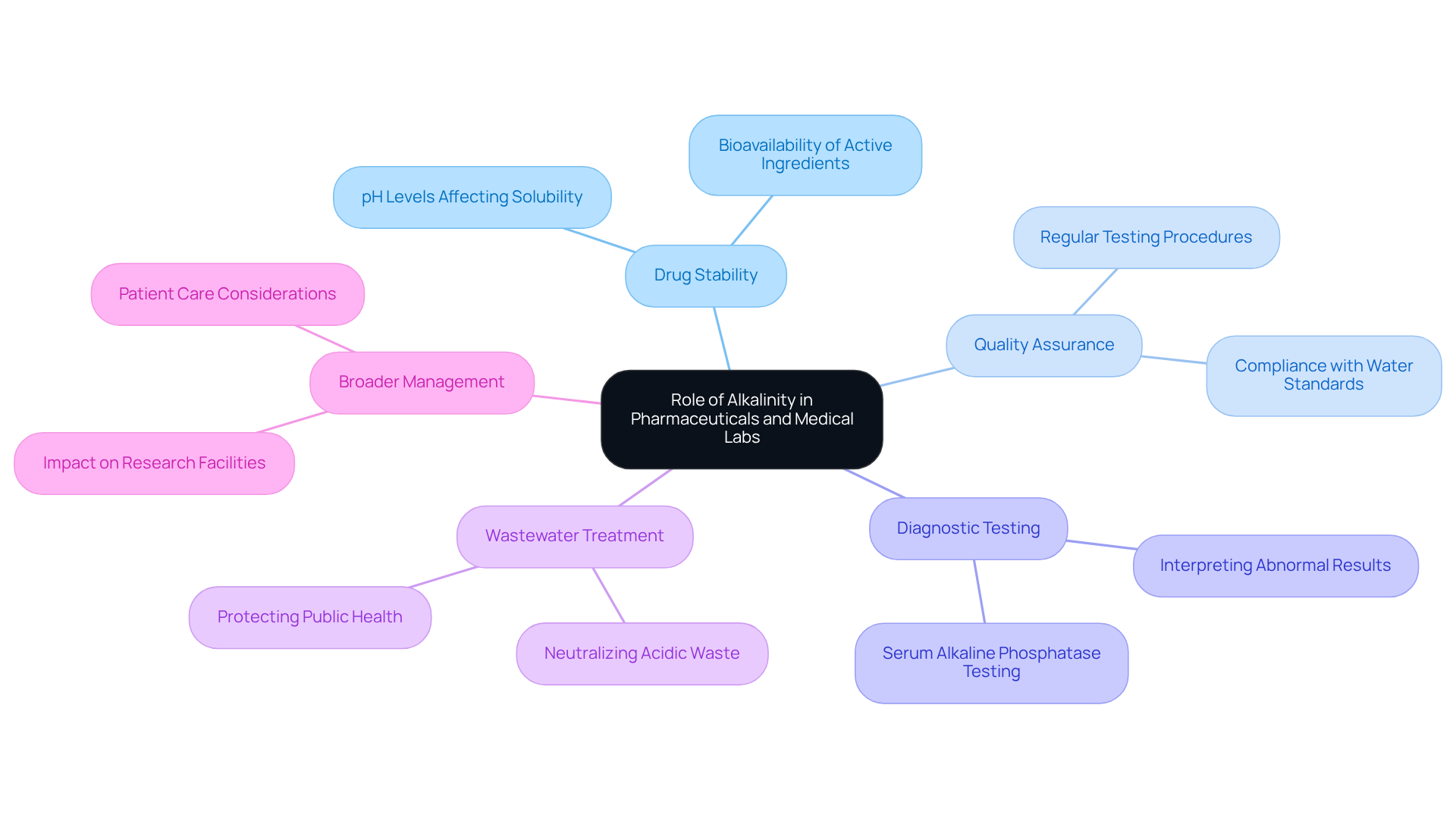
Trace the Historical Development of Alkalinity Measurement Techniques
The assessment of basicity has undergone significant development since its inception, which primarily relied on titration methods. This initial approach involved the addition of a known acid to a water sample until a specific pH endpoint was reached. As the field advanced, more sophisticated techniques emerged, including potentiometric titration and colorimetric analysis, which greatly improved precision and efficiency. Notably, the introduction of automated titration systems has revolutionized the process, enabling rapid and accurate evaluations of in high-throughput testing environments.
Recent advancements have shown that these automated systems can reduce analysis time by as much as 50%, allowing facilities to handle larger sample volumes without compromising accuracy. Understanding these historical developments is essential for appreciating the sophisticated methodologies utilized in contemporary laboratories, which continue to evolve in response to the increasing demands for precision in scientific research.
Furthermore, initiatives such as the 'Carbon to Sea Initiative' underscore the practical applications of these advancements, aiming to monitor what does alkalinity measure in water chemistry and contributing to environmental science. Experts in the field emphasize the significance of these advancements, asserting that 'the evolution of measurement techniques is vital for addressing contemporary challenges in environmental monitoring.
Conclusion
Understanding alkalinity is essential for maintaining the delicate balance of pH levels in various environments, particularly in laboratory settings. Alkalinity measures the water's ability to neutralize acids, which is crucial for stabilizing pH levels and protecting both aquatic life and chemical processes. The significance of alkalinity extends beyond simple measurement; it plays a vital role in pharmaceutical and medical laboratories, ensuring the efficacy and safety of drug formulations and patient care.
Key insights discussed include the relationship between alkalinity and pH, where a stable pH is often a sign of elevated alkalinity, essential for numerous chemical reactions and biological processes. The article also highlights the historical evolution of alkalinity measurement techniques, from basic titration methods to advanced automated systems that enhance precision and efficiency in testing. This evolution reflects the growing importance of accurate alkalinity assessments in various scientific and environmental contexts.
In conclusion, the importance of alkalinity in laboratory testing cannot be overstated. It not only contributes to the integrity of pharmaceutical products and environmental health but also underscores the need for ongoing advancements in measurement techniques. Emphasizing the significance of alkalinity will foster a deeper understanding of its impact on scientific research, healthcare, and environmental monitoring, encouraging professionals to prioritize precise alkalinity measurements in their work.




