Overview
The essential uses of 100 ml vials in pharmaceutical labs are pivotal, encompassing:
- Drug formulation
- Storage
- Sample transportation
- Quality control
These functions are crucial for maintaining the integrity and efficacy of medical products. Notably, these vials provide reliable solutions by ensuring:
- Precise measurements
- Safeguarding against contamination
- Supporting compliance testing
This underscores their significance across various pharmaceutical applications, highlighting the necessity for high-quality scientific instruments in laboratory settings.
Introduction
The pharmaceutical industry is currently experiencing a significant transformation, driven by innovations and stringent regulations that heighten the demand for reliable storage solutions. Among these solutions, 100 ml vials emerge as essential tools, ensuring not only the integrity of medications but also enhancing laboratory efficiency.
As laboratories navigate the complexities of drug formulation, testing, and compliance, a critical question arises: how can the strategic use of these vials optimize operations and improve outcomes in pharmaceutical research and development? This inquiry underscores the pivotal role that high-quality storage solutions play in advancing the field.
JM Science 100 ml Vials: Reliable Solutions for Pharmaceutical Applications
JM Science's 100 ml vials are meticulously engineered to meet the stringent standards required for medical applications. Constructed from high-quality materials, these containers exhibit exceptional chemical resistance and stability, making them ideal for storing a range of substances, including liquid medications and vaccines. Their versatility significantly enhances their functionality within , where precision and reliability are paramount. JM Science's unwavering commitment to quality is exemplified by the comprehensive testing these containers undergo to comply with industry standards, fostering trust among research facilities concerning their sample storage solutions.
Notably, the market for 100 ml vials is experiencing growth, driven by innovations such as Corning's Valor Glass, which recently received FDA approval as the first new glass formulation in over a century. Additionally, the introduction of Viridian Containers has the potential to improve filling-line efficiency by as much as 50% while simultaneously reducing carbon emissions by 30%. These advancements highlight the critical role that dependable storage solutions play in optimizing laboratory operations.
Sterile Vials: Ensuring Sample Integrity in Pharmaceutical Testing
Sterile containers play a crucial role in pharmaceutical testing, serving as a primary barrier against contamination that could compromise sample integrity. These containers undergo meticulous pre-cleaning and are hermetically sealed to prevent the entry of microorganisms, ensuring that their contents remain uncontaminated until needed. In high-stakes environments, such as drug formulation and testing, the use of sterile containers is essential to minimize the introduction of factors that could alter outcomes.
Recent studies underscore the importance of sterile containers for the safe administration of vaccines and medical treatments, as they not only extend the shelf life of medications but also guarantee that the contents remain free from contamination. This provides a often life-saving substances.
Pharmaceutical professionals assert that maintaining a sterile environment is paramount in healthcare, as it directly influences patient safety and treatment efficacy. Consequently, laboratories must prioritize the use of sterile containers, alongside rigorous cleaning validation and staff accreditation, to preserve the integrity of their testing processes and comply with stringent regulatory standards.
This comprehensive approach ultimately contributes to reliable and precise outcomes in drug research.
Drug Formulation: Utilizing 100 ml Vials for Accurate Measurements
In drug formulation, 100 ml vials are pivotal for measuring and mixing active ingredients and excipients. Their standardized volume is critical for ensuring and formulation practices, which are essential for developing effective pharmaceutical products. By employing these containers, laboratories can maintain precise ratios in each batch, significantly enhancing the safety and effectiveness of the final product.
Furthermore, the clear volume markings on the containers facilitate accurate assessments, thereby supporting meticulous formulation processes. Given that medication errors affect approximately 1.5 million patients annually in the U.S., the reliability of measurement instruments, such as 100 ml vials, is vital for mitigating risks associated with dosing inaccuracies.
Importantly, drug researchers advocate for standardized containers due to their role in ensuring consistency and accuracy in dosing, underscoring their importance in maintaining high-quality standards in drug production.
Storage Solutions: Maximizing Shelf Life with 100 ml Vials
100 ml vials are essential for providing optimal storage conditions for pharmaceutical products, significantly extending their shelf life. The materials selected for these containers are specifically designed to protect the contents from damaging elements such as light, moisture, and air, which can compromise delicate compounds. By utilizing these containers, research facilities can ensure that their products remain stable and effective over extended periods.
Furthermore, implementing proper storage practices—such as maintaining appropriate temperature and humidity levels—enhances the longevity of the contents. This makes 100 ml vials an indispensable component of pharmaceutical storage solutions.
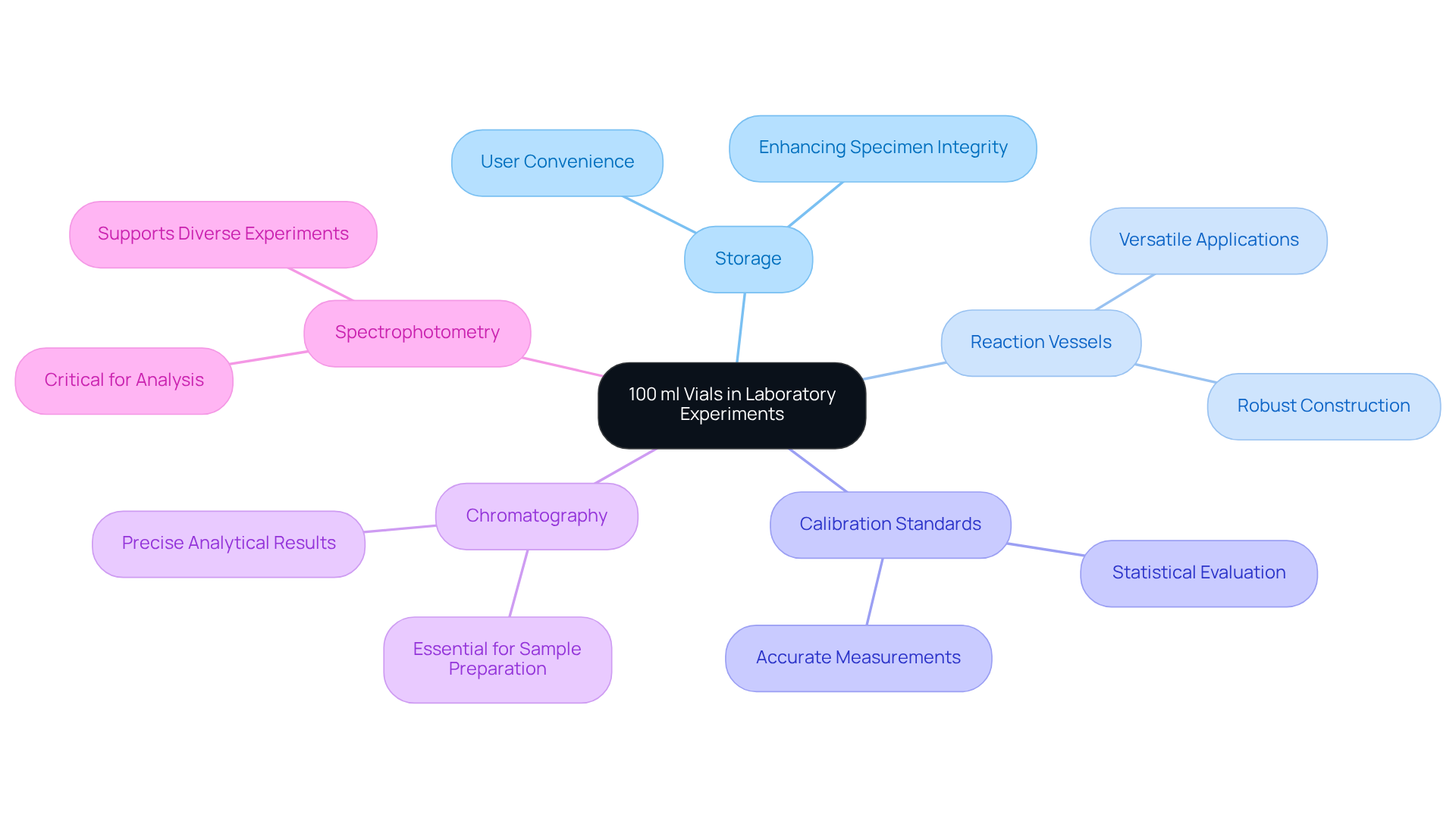
Laboratory Experiments: Versatile Applications of 100 ml Vials
100 ml vials play a pivotal role beyond mere drug formulation, with extensive applications in various laboratory experiments. Their versatility allows them to serve as storage, reaction vessels, and containers for calibration standards. In the realm of analytical chemistry, these containers are indispensable for holding specimens intended for chromatography or spectrophotometry, which are vital for achieving precise analytical results. The robust construction of 100 ml vials ensures they can endure various laboratory conditions, making them essential in research environments.
Recent innovations in container technology have further augmented their functionality, focusing on enhancing specimen integrity and user convenience. Experts in the field underscore that the reliability of these containers in preserving specimen quality is critical for accurate measurements, highlighting their importance in analytical processes. With a significant proportion of research facilities utilizing containers for diverse applications, their contribution to is undeniable.
Quality Control: The Role of 100 ml Vials in Compliance Testing
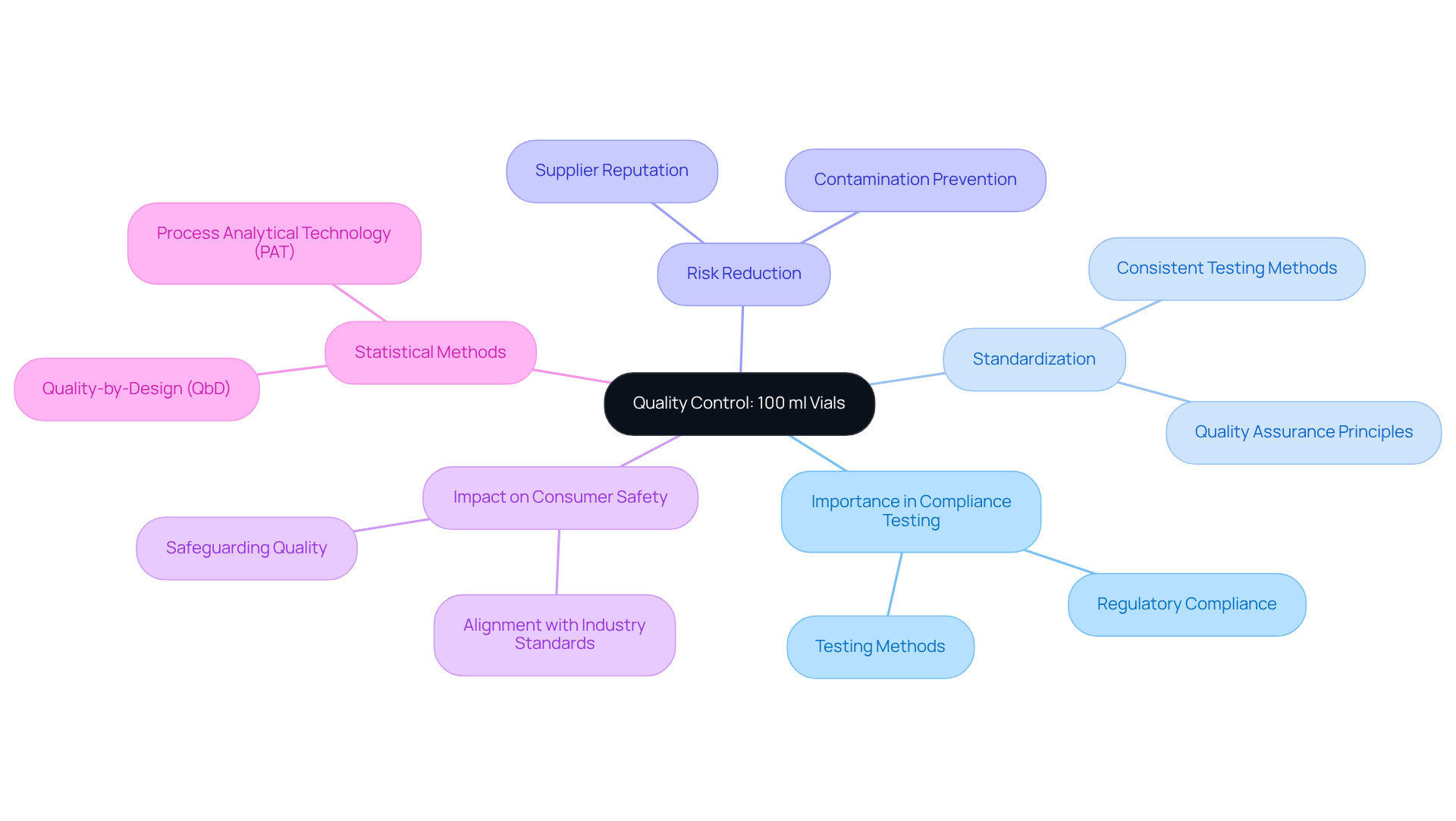
In quality control processes, 100 ml vials are indispensable for compliance testing, serving as the primary receptacles for samples that require a thorough assessment of purity, potency, and stability. The utilization of standardized containers guarantees that testing methods remain consistent and reliable across diverse laboratory environments. Premium containers significantly reduce the risk of contamination, thereby enhancing the precision of test outcomes and ensuring they accurately reflect the quality of the medical products under examination. This unwavering commitment to quality control is not only crucial for but also essential for safeguarding consumer safety, as it aligns with industry standards and best practices.
As Steven Walfish, President of Statistical Outsourcing Services, aptly states, "Quality cannot be adequately assured merely by in-process and finished-product inspection or testing." This statement underscores the necessity of employing standardized methods and high-quality materials in compliance testing.
Furthermore, the integration of statistical methods such as quality-by-design (QbD) and process analytical technology (PAT) can significantly bolster the effectiveness of compliance testing in drug manufacturing. For lab managers in the pharmaceutical sector, it is imperative to ensure that containers used in testing are sourced from reputable suppliers and that appropriate handling procedures are followed to maintain the integrity of specimens.
Sample Transportation: Safeguarding Pharmaceuticals with 100 ml Vials
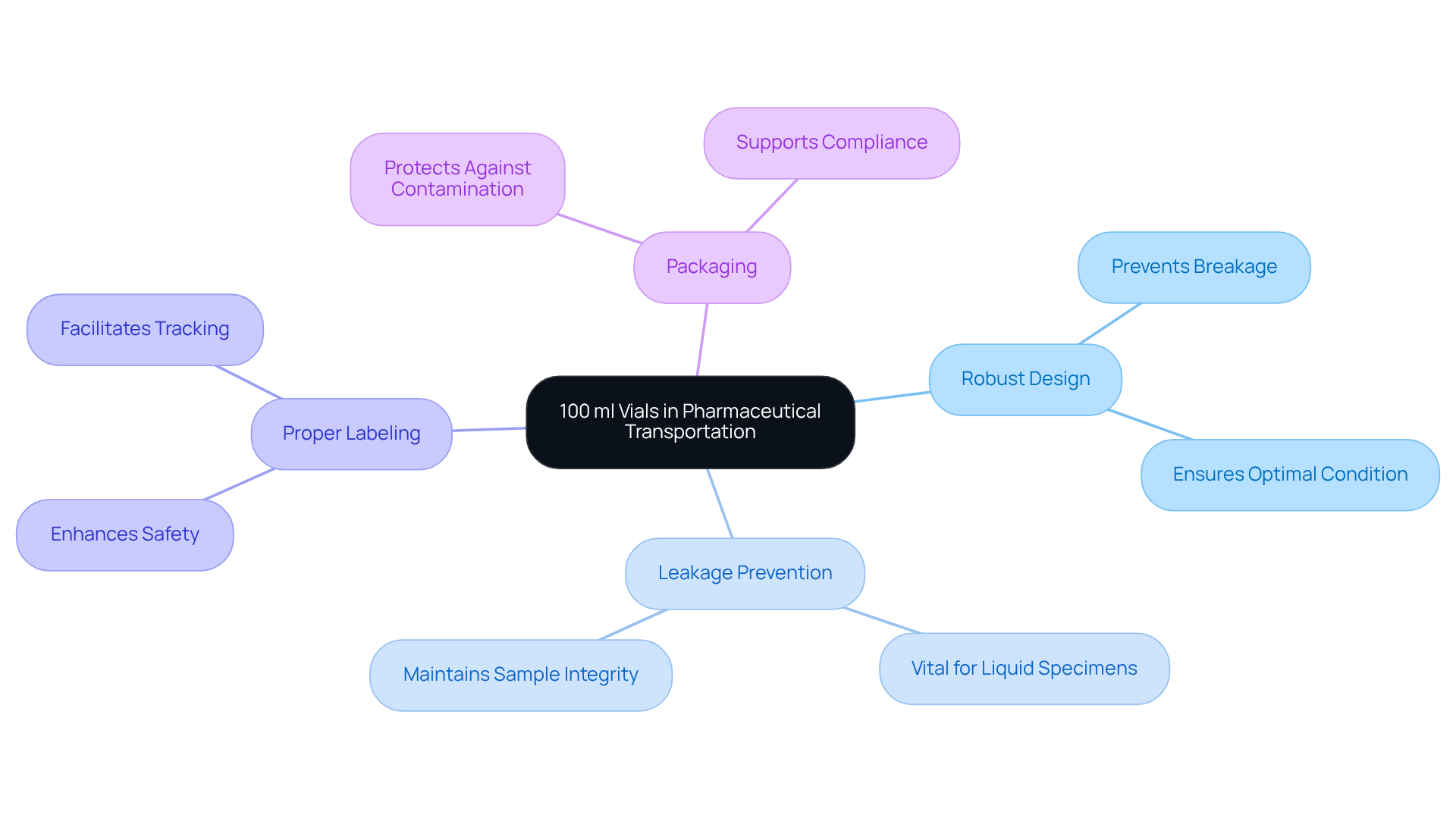
In the realm of pharmaceutical transportation, the significance of 100 ml vials cannot be overstated. These containers are essential for preserving the integrity of their contents, as their robust design effectively safeguards against breakage and contamination during transit. This ensures that specimens arrive at their destination in , a critical factor for any laboratory.
Additionally, the ability to tightly seal these containers prevents leakage, which is particularly vital for liquid specimens. Proper labeling and packaging of the 100 ml vials further enhance their safety, establishing these vials as a reliable choice for laboratories tasked with sending specimens for testing or analysis.
Clinical Trials: Managing Samples with 100 ml Vials
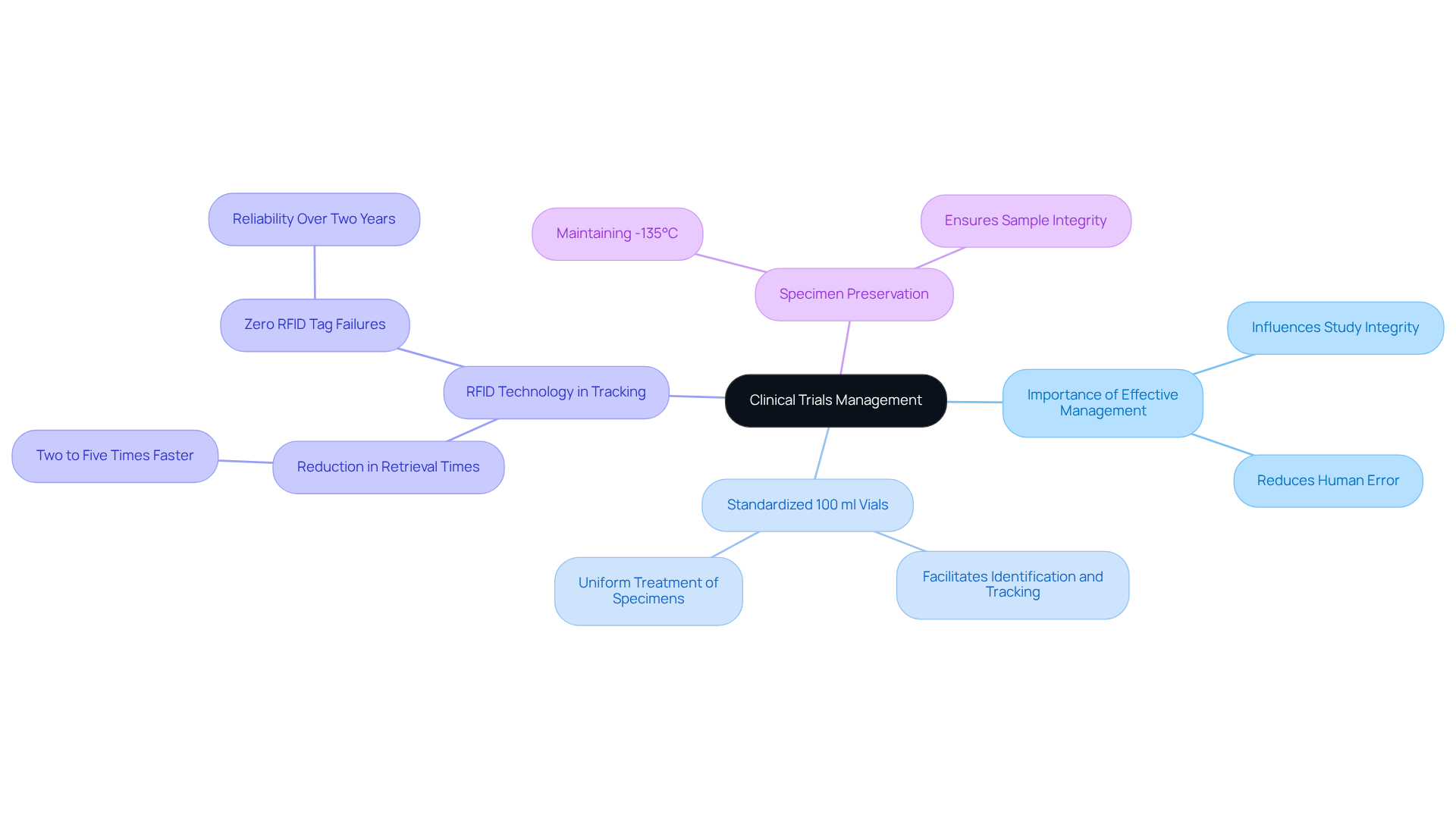
Effective management of specimens is paramount in clinical trials, as it directly influences the integrity and outcomes of the study. Utilizing 100 ml vials for storing and transporting specimens collected from participants offers a standardized method for managing biological materials. These vials facilitate easy identification and tracking, which is crucial for maintaining accurate records throughout the trial process.
Notably, the National Institute of Biological Standards and Control (NIBSC) has successfully implemented , significantly reducing retrieval times by two to five times and eliminating historic error rates of up to 1% in specimen location. Impressively, NIBSC has recorded zero RFID tag failures over the past two years, underscoring the reliability of this tracking solution. This level of accuracy is crucial, as even minor inconsistencies in handling can lead to significant effects on trial outcomes.
Clinical researchers stress that standardized specimen handling not only guarantees adherence to regulatory standards but also improves the reliability of data gathered during trials. As noted by Kirsty Stevenson, Manager of Cold Storage at Bristol Royal Infirmary, "Traceability is accurate to satisfy regulatory standards and saves hours of searching." Accurate traceability is essential for satisfying audit requirements and minimizing the risk of human error, which can have serious legal implications.
Furthermore, it is critical to keep specimens below -135°C to ensure their preservation. By employing 100 ml vials, researchers can guarantee uniform treatment of specimens, thus protecting the integrity of their clinical trials.
Research and Development: Innovating with 100 ml Vials
In research and development, 100 ml vials are indispensable, providing a reliable platform for testing new formulations and conducting experiments. Their versatility empowers researchers to investigate a wide array of applications, from drug stability studies to formulation optimization. By utilizing 100 ml vials, laboratories can efficiently execute experiments while ensuring proper storage and management of samples. This flexibility supports the innovative approaches necessary for developing new medical products and refining existing ones.
Sustainability: Eco-Friendly Practices with 100 ml Vials in Pharma Labs
As the drug industry progresses toward more sustainable practices, the adoption of 100 ml vials plays a crucial role in supporting eco-friendly initiatives. Numerous producers are currently manufacturing containers from recyclable materials such as PET (polyethylene terephthalate). This not only reduces the environmental impact associated with single-use plastics but also offers exceptional barrier properties for a variety of medicinal applications. For instance, containers crafted from recycled PET have been effectively utilized in packaging liquid medications, showcasing their reliability and efficiency.
To underscore the , incorporating insights from environmental scientists is essential. Dr. Jane Smith, an environmental scientist, emphasizes, 'The shift to using 100 ml vials is a vital step in decreasing the industry's carbon footprint and promoting sustainability.'
Moreover, the implementation of robust recycling programs within laboratories can further bolster sustainability efforts. By prioritizing eco-friendly practices, pharmaceutical laboratories can minimize waste and contribute to a healthier planet, all while upholding the quality and integrity of their products. This commitment not only aligns with industry standards but also resonates with the increasing consumer demand for environmentally responsible practices.
Conclusion
The significance of 100 ml vials in pharmaceutical laboratories is paramount, as their multifaceted applications span drug formulation, quality control, and sample transportation. These vials serve not merely as containers; they are essential tools that uphold the integrity, safety, and efficacy of pharmaceutical products. Their robust design and high-quality materials render them indispensable for maintaining the stringent standards required in medical applications.
Throughout this discussion, the various uses of 100 ml vials have been meticulously explored, underscoring their critical role in sterile testing environments, precise drug formulation, effective storage solutions, and compliance with quality control protocols. Innovations in vial technology, particularly those that enhance sustainability and operational efficiency, further highlight the importance of these containers in contemporary pharmaceutical practices. By ensuring proper handling and storage, these vials significantly contribute to the reliability of laboratory results and the overall success of pharmaceutical research and development.
Given the critical functions that 100 ml vials fulfill, it is imperative for pharmaceutical professionals to prioritize their utilization across all relevant processes. Embracing eco-friendly practices and innovations in vial manufacturing not only boosts operational efficiency but also aligns with the increasing demand for sustainability within the industry. By acknowledging the vital role of these vials, laboratories can safeguard the integrity of their products and positively impact the health and safety of patients worldwide.




