Overview
This article presents a comprehensive step-by-step guide on effectively interpreting chromatography graphs. Understanding the axes, baseline, peaks, retention times, and calibration curves is crucial for accurate data interpretation. These elements collectively inform the identification and quantification of compounds in analytical chemistry, underscoring their importance in laboratory settings. Mastery of these components not only enhances analytical skills but also elevates the quality of scientific inquiry. By grasping these fundamentals, readers can significantly improve their analytical capabilities and contribute to more precise scientific outcomes.
Introduction
In the realm of analytical chemistry, chromatography emerges as an indispensable technique for the separation and analysis of complex mixtures. This powerful method hinges on the interaction between a stationary phase and a mobile phase, enabling scientists to isolate individual components based on their distinct properties. Techniques such as High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) empower researchers to address a wide array of applications, ranging from pharmaceutical testing to environmental monitoring.
Understanding the fundamental principles and components of chromatography, alongside mastering the interpretation of chromatograms, is paramount for achieving accurate and reliable results. As the field continues to evolve, it is essential for professionals to remain informed about the latest advancements and best practices. This commitment not only enhances the effectiveness of data analysis in laboratories but also reinforces the importance of high-quality scientific instruments in achieving optimal outcomes.
Understanding Chromatography: Key Principles and Techniques
Chromatography serves as a cornerstone analytical technique, proficiently separating complex mixtures into their individual components. This separation process relies on two distinct phases: the stationary phase, which remains fixed, and the mobile phase, which traverses through the stationary phase. The effectiveness of this technique is rooted in the varying interactions of the components with these phases, leading to their separation based on distinct properties.
- High-Performance Liquid Chromatography (HPLC): This method employs a liquid mobile phase to achieve separation based on the differential interactions of compounds with the stationary phase. HPLC is particularly esteemed for its precision and capability to analyze a wide array of substances, making it indispensable in pharmaceutical laboratories. JM Science Inc. offers an extensive selection of , featuring Shodex, CapcellPak, and Reprosil, tailored for all scales of high-performance liquid separation. Additionally, JM Science provides essential HPLC fittings, manual injection valves, and solvent reservoir kits, all available at exceptionally competitive prices.
Recent advancements in HPLC techniques have further enhanced its capabilities, allowing for more efficient evaluations and improved resolution of complex mixtures. Dr. Sujatha Mahadevarao Premnath emphasizes the importance of HPLC in genetic analysis, stating that denaturing high-performance liquid separation reliably detects BRCA1 and BRCA2 mutations, underscoring its essential role in pharmaceutical applications.
- Gas Chromatography (GC): Utilizing a gaseous mobile phase, GC is optimal for the analysis of volatile substances. This method is commonly applied in various areas, such as environmental testing and food safety, due to its efficiency in isolating and measuring volatile compounds.
Understanding how to interpret a chromatography graph, which illustrates signal strength over time or volume, is crucial for analyzing chromatograms and comprehending the outcomes of this analytical technique. Each peak in a chromatogram corresponds to a specific compound, with its height and area providing insights into the concentration and purity of the substances analyzed. Acceptance criteria for transition ratios typically fall within 20% of the mean of the calibrators used in assays, highlighting the importance of accuracy in these measurements and their direct relevance to chromatogram analysis.
Real-world applications of HPLC extend beyond genetic testing; a modified methodology for measuring THC-COOH has been developed to mitigate interferences from isobaric compounds. This advancement is particularly significant in pharmaceutical contexts, where accurate measurement is essential for compliance and safety. JM Science's innovative HPLC solutions, featuring high-performance columns and fittings, support these essential applications. Furthermore, a case study on real-time documentation for root-cause examination in LC-MS/MS workflows illustrates the importance of meticulous record-keeping in separation processes. This practice not only aids in the swift recognition and correction of problems but also enhances overall quality assurance and reliability in pharmaceutical laboratory outcomes.
In summary, grasping the principles and applications of separation techniques is vital for effective data analysis in laboratories. The ongoing advancement of separation techniques, supported by JM Science's high-quality scientific instruments, ensures that they remain at the forefront of analytical chemistry, providing researchers with the essential tools for accurate and reliable measurements. For pharmaceutical lab managers, these advancements underscore the critical role of chromatography in ensuring the quality and safety of pharmaceutical products.
Types of Chromatography: Gas vs. Liquid and Their Graphical Representations
Chromatography stands as a fundamental analytical technique, categorized primarily into two types: Gas Chromatography (GC) and Liquid Chromatography (LC).
Gas Chromatography (GC) is predominantly employed for analyzing volatile compounds. In a GC chromatogram, signals illustrate the duration each component takes to emerge from the column, with the x-axis typically representing duration and the y-axis reflecting detector response. The resolution and separation efficiency of GC can be influenced by various factors, including column type and temperature settings. Recent studies indicate that GC methods may encounter limitations in separating certain isomers, potentially impacting result accuracy.
Liquid Chromatography (LC), in contrast, is utilized for non-volatile compounds and excels in handling complex mixtures. The chromatogram produced by LC also displays elevations, but retention durations can significantly differ from those observed in GC due to the unique characteristics of the mobile phase. A notable case study, "," showcased the effectiveness of LC/MS-MS in quantifying anilines and their derivatives, emphasizing its advantages over GC methods, particularly regarding selectivity and speed. This research concluded that LC/MS-MS could serve as a cost-effective option for routine laboratory testing, especially as it prevents co-elutions that complicate GC outcomes.
Furthermore, an interlaboratory comparison involving ten accredited laboratories assessed the detection of anilines and their derivatives, with results evaluated according to ISO 13528. This evaluation process underscores the reliability of findings related to LC/MS-MS.
Understanding these differences is essential for accurate chromatogram interpretation, as each technique yields distinct peak shapes and retention times. For instance, the examination of the ω-6/ω-3 ratio in various samples revealed ratios of approximately 17.3 and 18.6, illustrating how different methodologies can provide varying insights into compound behavior. As the field of separation techniques advances, mastering the interpretation of chromatography graphs and staying abreast of current trends and professional insights will enhance the ability to interpret chromatograms effectively. This ensures that laboratory managers can make informed decisions based on trustworthy data.
As noted by Hannah Holtkamp, "Capillary electrophoresis in metallodrug development" emphasizes the importance of understanding various chromatographic techniques in advancing analytical capabilities.
Components of a Chromatography System: From Solvent to Detector
A typical chromatography system consists of several essential components that collaborate to achieve effective separation and analysis of compounds:
- Solvent Reservoir: This crucial component holds the mobile phase, which is vital for transporting the sample through the system. The choice of solvent can significantly influence the separation process and the overall results.
- Pump: Responsible for delivering the mobile phase at a controlled flow rate, the pump ensures consistent flow, which is vital for maintaining separation efficiency and resolution. Fluctuations in flow can lead to inaccurate results.
- Column: Often regarded as the heart of the chromatography system, the column is where the actual separation of compounds occurs. The selection of the column type and its dimensions directly impact the resolution and efficiency of the separation process. For instance, a column with a higher resolution value can effectively distinguish maxima that overlap by as little as 2% of the total area, underscoring the significance of column selection in analytical applications.
- Detector: This component measures the response of the separated analytes as they elute from the column, generating the chromatogram. Various detectors, including UV-Vis, fluorescence, and mass spectrometry, are employed, each offering unique advantages depending on the specific analysis requirements.
Understanding these components is crucial for troubleshooting and optimizing chromatographic methods. A case study on how to read a chromatography graph emphasizes the importance of peak height, area, and retention time in assessing the composition and concentration of compounds. Effective interpretation of chromatographic data, including how to read a chromatography graph, is vital for accurate identification and quantification of analytes, reinforcing the necessity of mastering these principles in laboratory settings.
Furthermore, recent advancements in separation analyzers have significantly improved analytical capabilities, enabling more sensitive and specific detection of compounds. As Kazi Hasan, an experienced professional in the pharmaceutical sector, observes, "The incorporation of sophisticated separation techniques is vital for guaranteeing the precision and dependability of pharmaceutical analyses." This highlights the essential function of separation techniques in the pharmaceutical sector.
Moreover, effective communication and training among healthcare professionals can significantly improve results when utilizing separation techniques in medical diagnostics. As the market for separation detectors continues to expand, with a substantial portion controlled by advanced technologies, staying updated on these developments is crucial for laboratory managers seeking to enhance their separation setups. Expert opinions underscore that can lead to enhanced outcomes in both research and medical diagnostics, showcasing the vital role of these components in achieving dependable results.
JM Science distinguishes itself through its dedication to quality and customer assistance, ensuring that laboratory managers have access to the latest innovations and resources essential for effective analysis.
Retention Time vs. Relative Retention Time: What You Need to Know
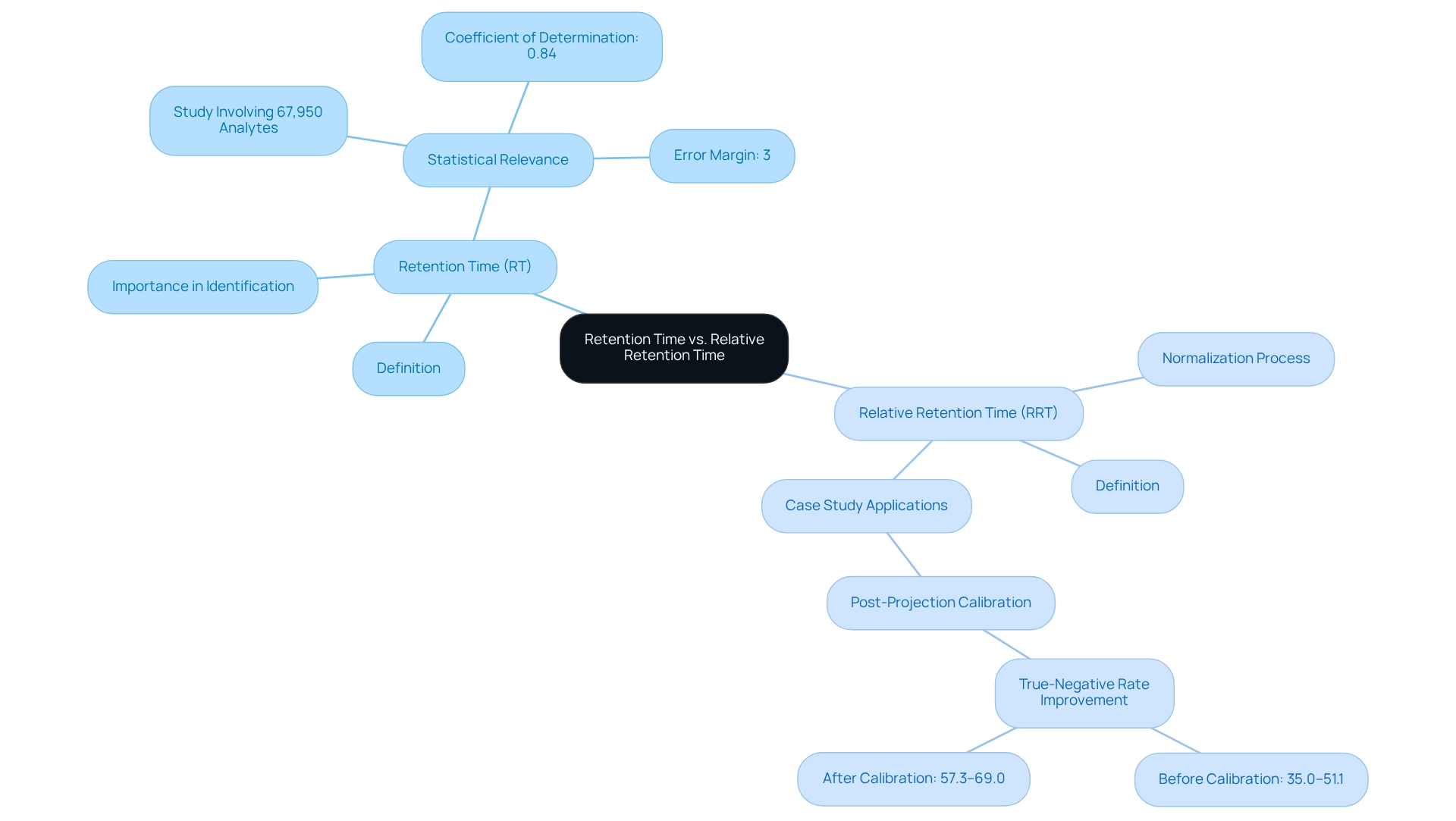
Retention duration (RT) is a fundamental metric in chromatography, defined as the period it takes for a compound to traverse the chromatography system and elute from the column. This parameter is crucial for the identification of compounds, as it provides a unique fingerprint for each analyte under specific conditions. Typically measured in minutes or seconds, RT serves as a benchmark for evaluating the behavior of various substances during examination.
Conversely, relative retention duration (RRT) is computed as the ratio of the retention duration of an analyte to that of a reference compound, expressed mathematically as RRT = RT of analyte / RT of reference. This normalization process is essential for comparing results across different chromatographic runs, allowing for a more consistent interpretation of data.
The importance of retention duration extends beyond simple identification; it plays a crucial role in ensuring the precision and dependability of chromatographic analyses. Recent studies involving 67,950 proprietary analytes have demonstrated impressive coefficients of determination of 0.84, with a mere 3% error margin in retention duration measurements. Such statistics underscore the significance of accurate retention duration calculations in achieving .
Furthermore, the concept of relative retention duration has gained traction in modern separation techniques. A recent case study titled 'Filtering and Ranking of Putative Candidates Using Post-Projection Calibration' illustrates the application of RRT in enhancing the precision of filtering unknown identities in untargeted evaluations. The study revealed that post-projection calibration improved true-negative rates from 35.0%–51.1% before calibration to 57.3%–69.0% afterward, highlighting the effectiveness of RRT in refining candidate identification.
As the field of chromatography advances, the necessity for a comprehensive retention duration database for heterogeneous molecules has become increasingly evident. Such a resource would not only enhance prediction accuracy but also facilitate collaboration among laboratories, fostering advancements in analytical methodologies. This need is echoed in the research community, where all authors critically evaluated and approved the manuscript, emphasizing the collective importance of these findings.
In summary, understanding how to read a chromatography graph, along with grasping retention time and relative retention time, is essential for the precise evaluation and interpretation of chromatographic data. By adhering to current best practices for measuring these parameters, laboratories can enhance their analytical capabilities and ensure the reliability of their results. JM Science Inc. supports these initiatives by supplying high-quality HPLC columns and accessories, including the Shodex and CapcellPak series, which are designed for optimal performance and accuracy in chromatographic evaluation.
Additionally, JM Science offers competitive pricing on HPLC fittings and solvent reservoir kits, ensuring that laboratories have access to high-quality tools at exceptional value.
Interpreting Calibration Curves: A Guide to Accurate Analysis
Calibration curves are essential graphical tools that illustrate the relationship between the concentration of an analyte and its corresponding detector response. Understanding these curves is crucial for achieving precise quantitative evaluations in separation science. Below is a step-by-step approach to creating and utilizing calibration curves effectively:
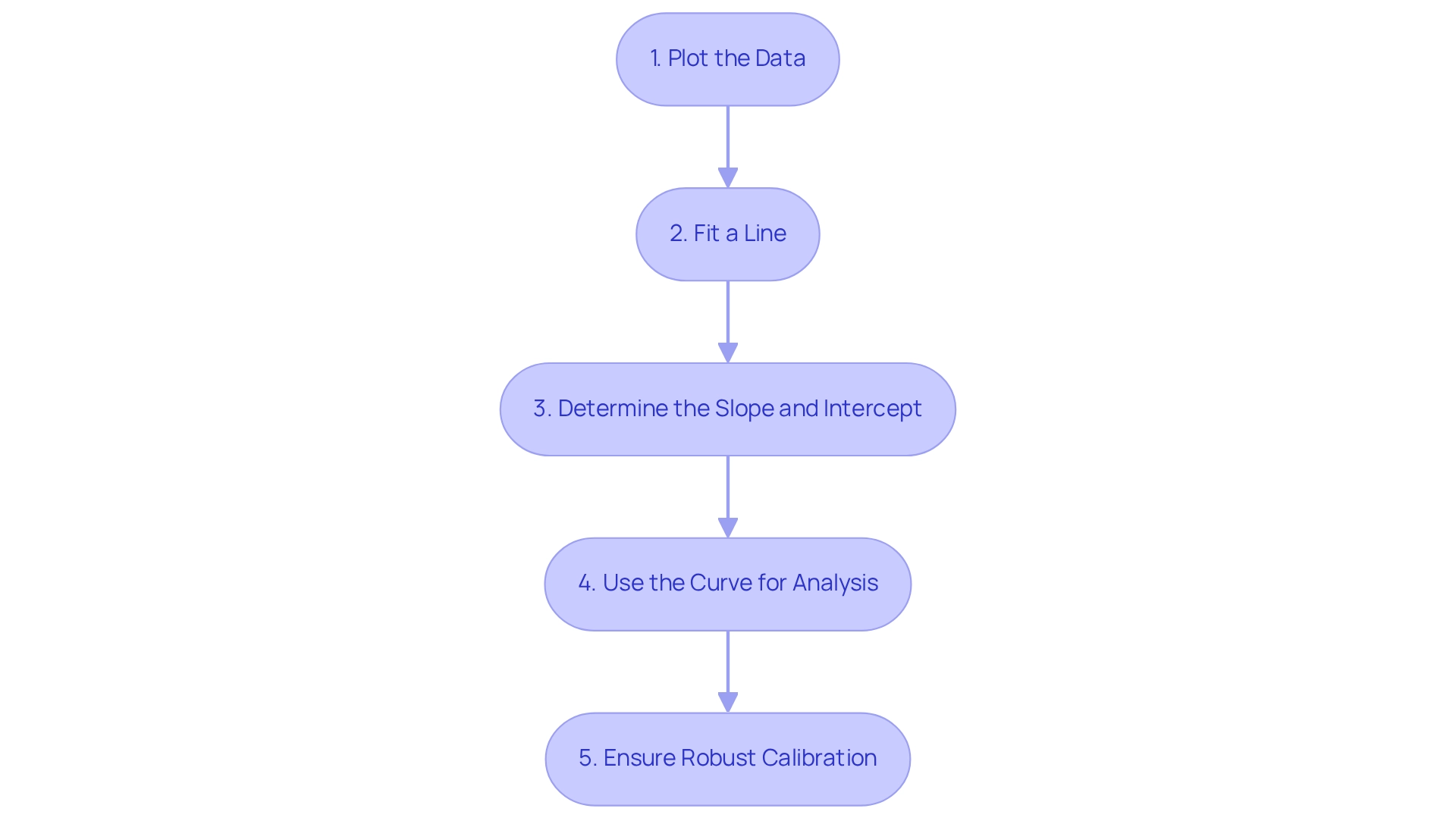
- Plot the Data: Start by using known concentrations of the analyte to create a scatter plot, with concentration on the x-axis and detector response on the y-axis. This visual representation lays the groundwork for further examination.
- Fit a Line: Apply linear regression to the plotted data points to establish a calibration line. This line is vital, as its equation enables the calculation of unknown concentrations based on their detector responses.
- Determine the Slope and Intercept: The slope of the calibration line indicates the sensitivity of the method, while the intercept should ideally be zero to ensure accurate quantification. A well-defined slope signifies a reliable response to concentration changes, which is essential in analytical applications.
- Use the Curve for Analysis: Measure the detector response of unknown samples and utilize to determine their concentrations. This process is fundamental in various analytical scenarios, particularly in bioanalytical measurements where precision is paramount.
The significance of calibration curves in analytical chemistry is profound. Recent advancements underscore the necessity of including at least six non-zero samples to cover the intended calibration range, thereby ensuring robust and reliable data. Moreover, current trends advocate for the use of matrix-matched calibrators and stable isotope-labeled internal standards to enhance calibration accuracy in complex matrices, as highlighted in recent studies on optimal calibration strategies in LC-MS/MS bioanalytical measurement procedures.
Real-world applications of calibration curves are exemplified in studies such as the photocatalytic removal of levonorgestrel, where the effectiveness of the method was quantitatively assessed using calibration techniques. The research demonstrated over 90% removal efficiency, underscoring the practical implications of accurate calibration in environmental monitoring and public health. As noted by Cheng WL, "Loh TP and Cheng WL researched the literature, conceived the study, and collected and analyzed the data," emphasizing the rigorous process behind ensuring accurate analytical methods.
In conclusion, mastering how to interpret chromatography graphs and develop calibration curves is vital for achieving accuracy in quantitative analysis within separation techniques. This knowledge ultimately supports progress in both research and healthcare.
Step-by-Step Guide to Reading Chromatography Graphs
To effectively read a chromatography graph, it is essential to follow a series of detailed steps.
- Identify the Axes: The x-axis typically represents time, while the y-axis indicates the detector response, which is crucial for understanding the data presented.
- Identify the Baseline: Recognizing the baseline of the graph is vital, as it indicates the absence of analytes and serves as a reference point for analyzing the highest values.
- Examine the Summits: Each summit corresponds to a distinct component in the sample. The height and area of the summit provide quantitative information about the analyte's concentration, enabling accurate assessments of sample composition. A chromatographic signal indicates the detector response to a separated analyte in a mixture, with the area or height of the signal often related to its concentration.
- Determine Retention Durations: It is important to record the moment at which each peak emerges; this is referred to as the retention duration for that component. For instance, in a recent analysis, displayed a notable concentration of benzene compounds around the 9-minute mark, underscoring the significance of retention duration in identifying substances. Understanding retention durations is essential, as it aids in verifying the identity of the analytes found in your sample.
- Contrast with Standards: Utilize established benchmarks to assess unfamiliar maxima based on their retention durations and shape profiles. This comparison is critical for confirming the identity of the analytes present in your sample.
- Check for Anomalies: Vigilantly look for any unusual shape patterns or unexpected retention times, which may indicate issues such as injection problems, overloaded columns, or stationary phase complications. For example, a divided peak may signify an injection problem or other analytical errors that warrant attention.
By adhering to these steps, readers can confidently analyze graph results and comprehend how to read a chromatography graph, facilitating the extraction of significant insights from their data. Additionally, existing training materials, such as instructional videos and application libraries, can further enhance understanding and skills in data analysis within this field. Real-world examples, such as the integration of mass spectrometry with gas analysis, illustrate the practical applications of these techniques in analytical chemistry, highlighting the importance of precise chromatogram interpretation.
Common Mistakes in Reading Chromatography Graphs and How to Avoid Them
When interpreting chromatography graphs, several prevalent mistakes can significantly impact the accuracy of results:
- Disregarding Baseline Noise: Baseline noise can obscure true maximum signals, leading to misinterpretation of maximum heights and areas. It is essential to carefully assess the baseline to distinguish between noise and actual signals.
- Misidentifying Summits: Confusing summits from different components is a common error that can result in erroneous conclusions. To mitigate this risk, always compare observed peaks with known standards to confirm their identity.
- Overlooking Retention Duration Variability: Retention durations can fluctuate due to factors such as temperature changes or variations in . Regular validation against standards is crucial to ensure accurate retention time assessments.
- Neglecting Calibration: Failing to utilize a calibration curve can lead to erroneous quantification of analytes. Regular calibration is vital to maintain accuracy in measurements and should be performed consistently.
- Assuming Linear Response: Not all detectors exhibit a linear response across all concentration ranges. Understanding the specific characteristics of the detector used is critical to avoid misinterpretation of results.
Statistics indicate that a notable proportion of chromatographic mistakes arise from these frequent traps, highlighting the necessity for caution in evaluation. Research has shown that misidentification of maxima can occur in over 30% of chromatographic evaluations, underscoring the significance of comprehensive training and adherence to best practices. Additionally, chromatograms have demonstrated a declining trend of oxygen levels from the initial injection to the final one over seven runs, illustrating how outcomes can differ considerably across various examinations.
Real-world examples further illustrate the consequences of misinterpretation. In one case study titled "Role of Chromatography in Drug Development and Quality Control," a pharmaceutical company faced product recalls due to incorrect peak identification, which compromised the integrity of their quality control processes. Such incidents emphasize the necessity of rigorous chromatographic training and the implementation of standard operating procedures.
Expert opinions from analytical chemists stress the importance of continuous improvement in the testing process. As D. Blumenthal noted, "The promotion of quality control and continuous improvement of the total testing process, including pre- and post-analytical phases, is essential for effective laboratory service."
By remaining vigilant about these common mistakes and adhering to current best practices, laboratory professionals can significantly enhance the reliability and accuracy of their chromatographic analyses. Furthermore, participating in gatherings such as the AOCS Annual Meeting & Expo on April 27, 2025, can provide valuable insights into current trends and practices in the field.
Best Practices for Analyzing Chromatography Graphs: Key Takeaways
To effectively analyze chromatography graphs, it is essential to implement the following best practices:
- Regular Calibration: Frequent updates to calibration curves are crucial for maintaining accuracy in measurements. A systematic review has shown that proper calibration procedures, including the use of matrix-matched calibrators and stable isotope-labeled internal standards, significantly enhance the reliability of results. In cases of strong curvature in calibration curves, alternative weighting factors like 1/y may be preferable.
- Use Standards: Always compare unknown samples against known standards. This practice not only confirms identifications but also helps establish a reliable relationship between instrument signals and analyte concentrations, which is vital for accurate data interpretation. As Oswald Sonntag, an independent consultant, advises, "Finally, our recommendation is to do a blanking first and measure at least two calibrators with two different concentrations covering the linear range in duplicates."
- Document Conditions: Keeping detailed records of experimental conditions is imperative, as variations can significantly impact results. This documentation aids in troubleshooting and ensures consistency across analyses.
- Review Data Thoroughly: Take the time to meticulously review chromatograms for anomalies or unexpected results. A single measurement is often associated with greater uncertainty compared to the average of multiple replicate measurements, underscoring the importance of thorough data evaluation.
- Stay Informed: Keeping abreast of advancements in separation techniques and technologies is essential for enhancing analytical capabilities. Engaging with current literature and industry news can provide insights into best practices and emerging methodologies. The publication suggests using third-party quality control materials to mitigate the risk of erroneous calibration curves and patient results.
By adhering to these best practices, laboratory professionals can enhance their proficiency in analyzing chromatography data and understand how to read a chromatography graph, leading to more reliable and accurate results. Additionally, the case study titled " highlights the importance of establishing a reliable relationship between instrument signals and analyte concentrations through proper calibration procedures.
Conclusion
Chromatography stands as an indispensable analytical technique, pivotal for the separation and analysis of complex mixtures across diverse fields, notably pharmaceuticals and environmental science. The contrast between High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) illustrates the adaptability of this method, facilitating customized approaches based on the characteristics of the compounds under scrutiny. A thorough understanding of chromatographic principles—such as retention time, relative retention time, and the formulation of calibration curves—is essential for precise data interpretation and dependable results.
Grasping the complexities of chromatography not only amplifies the effectiveness of laboratory analyses but also underscores the necessity for high-quality scientific instruments and ongoing education regarding advancements in the field. As the analytical landscape progresses, adopting best practices and remaining informed about emerging methodologies become crucial for laboratory managers and scientists alike.
Ultimately, a steadfast commitment to precision in chromatography enhances outcomes in both research and diagnostics, safeguarding the integrity of analytical results. By emphasizing education, comprehensive training, and the execution of robust analytical practices, laboratories can adeptly navigate the intricacies of chromatography with assurance, propelling advancements in scientific discovery and public health.




