Overview
This article outlines ten essential pH solutions vital for achieving accurate laboratory results. It underscores the significance of precision instruments, such as Karl Fischer titrators and Agilent pH meters, in conjunction with buffer solutions and calibration techniques. Collectively, these elements ensure reliable measurements and optimal performance across various scientific applications. By emphasizing the role of high-quality scientific instruments, the article aims to convey their importance in laboratory settings, ultimately fostering a deeper understanding of their impact on research outcomes.
Introduction
In the intricate realm of laboratory research, the precision of pH measurements can significantly influence the outcome of experiments, distinguishing between successful results and costly failures. As the reliance on accurate pH solutions continues to grow across diverse scientific disciplines, it becomes essential to grasp the fundamental tools and techniques that yield reliable results. How can laboratories effectively navigate the complexities of pH measurement to ensure their data remains both accurate and actionable? This article explores ten pivotal pH solutions designed to enhance measurement precision and streamline laboratory processes, ultimately reinforcing the integrity of scientific research.
JM Science Karl Fischer Titrators: Precision in pH Measurement
JM Science presents Karl Fischer titrators that deliver exceptional precision in moisture analysis, a critical factor for accurate pH measurement. These titrators are meticulously designed to minimize errors and enhance the , making them indispensable in environments focused on chemical and pharmaceutical research. Their cutting-edge technology guarantees the detection of even the slightest variations in moisture content, facilitating precise pH solutions for various applications. By integrating these titrators into your laboratory processes, you ensure the highest standards of accuracy and reliability in your analytical endeavors.
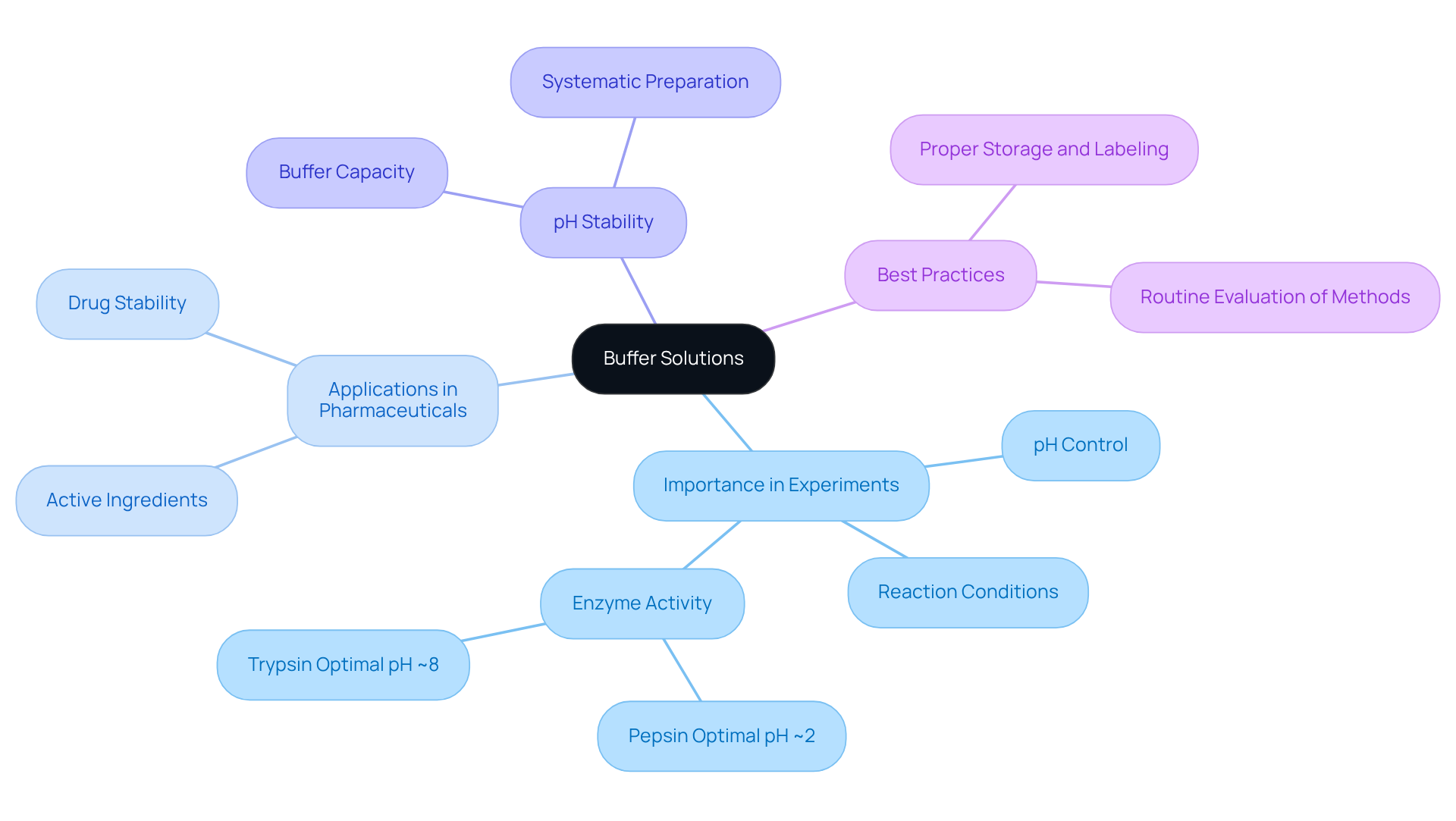
Buffer Solutions: Essential for pH Stability in Experiments
Buffer preparations are crucial for maintaining pH stability in laboratory experiments involving pH solutions. They effectively resist changes in pH solutions when acids or bases are introduced, ensuring that reactions occur under controlled conditions. In the pharmaceutical sector, pH solutions are particularly vital, as they enhance drug stability and manage reaction conditions, especially during the production of active pharmaceutical ingredients. Enzymes such as pepsin perform optimally at a pH of approximately 2, while trypsin operates best at around pH 8. This highlights the importance of selecting appropriate supporting substances for specific applications.
To achieve precise and reliable outcomes, laboratories must carefully select substances that align with their particular applications. This process involves understanding the desired pH range and selecting compatible weak acid/base couples based on the Henderson-Hasselbalch equation to create effective pH solutions. Consistent monitoring and adjustment of liquid mixtures are essential to ensure optimal performance; even minor variations can significantly impact experimental results. As biochemist Edward J. Fenn aptly noted, 'Proper preparation of aqueous mixtures is essential for mastering pH solutions in both chemical and biological experiments.'
Moreover, proper storage and labeling of mixtures, including the pH and preparation date, are vital for maintaining their effectiveness. Current trends indicate a growing emphasis on innovative support systems that cater to diverse research needs, including those aimed at enhancing flavor stability in food and beverage applications. To ensure the best outcomes, research facilities should routinely and adapt to new advancements in the field.
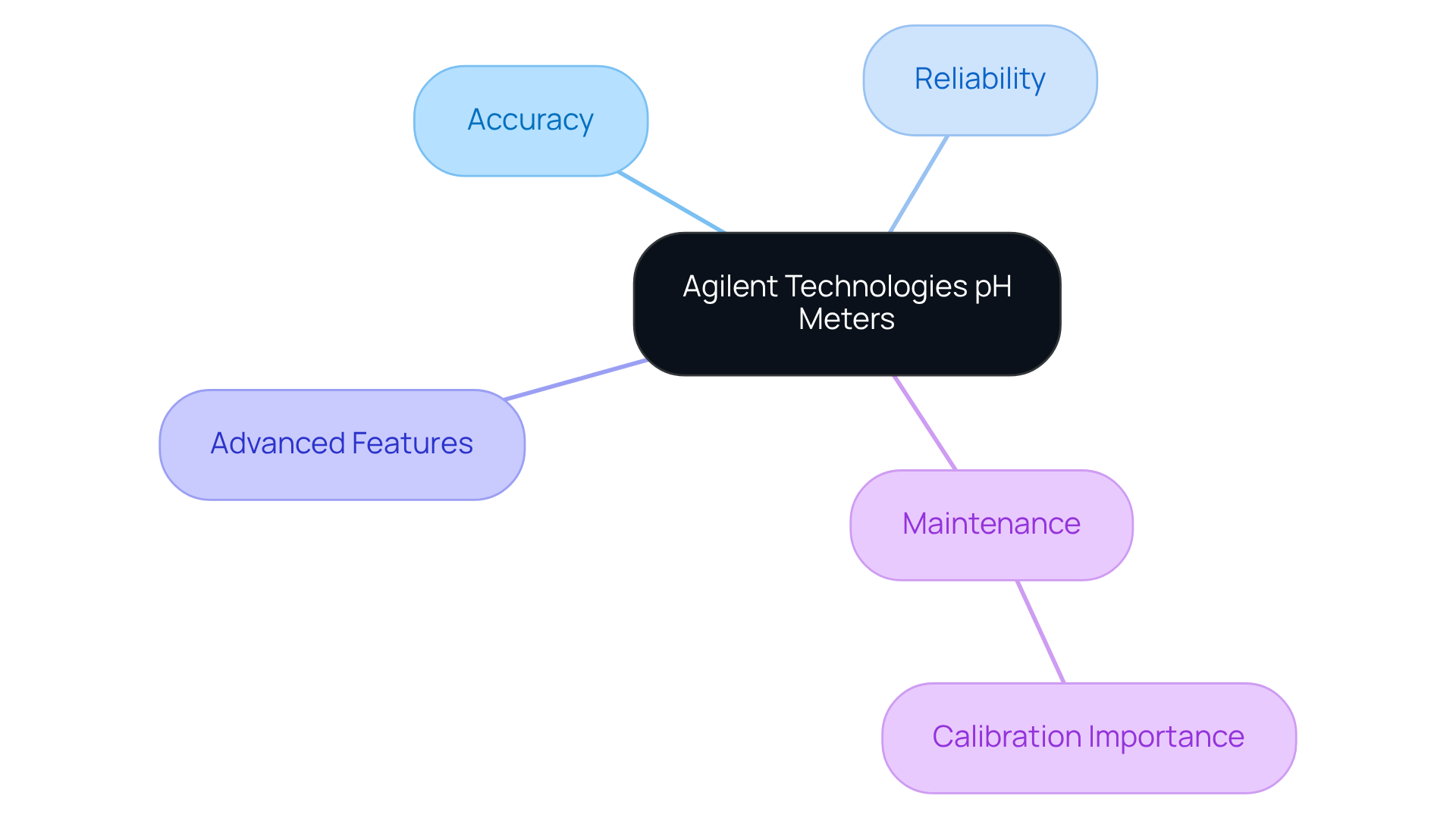
Agilent Technologies pH Meters: Accuracy for Analytical Applications
Agilent Technologies provides pH solutions that are celebrated for their exceptional accuracy and reliability in analytical applications. These sophisticated instruments are equipped with advanced features that facilitate precise assessments, making them indispensable for laboratories engaged in research and quality control.
To maintain their performance and accuracy across various experimental conditions, regular calibration and maintenance are not just recommended—they are essential. By investing in Agilent's pH solutions, laboratories can ensure they meet the highest standards of scientific excellence.
pH Calibration Solutions: Ensuring Reliable Measurements
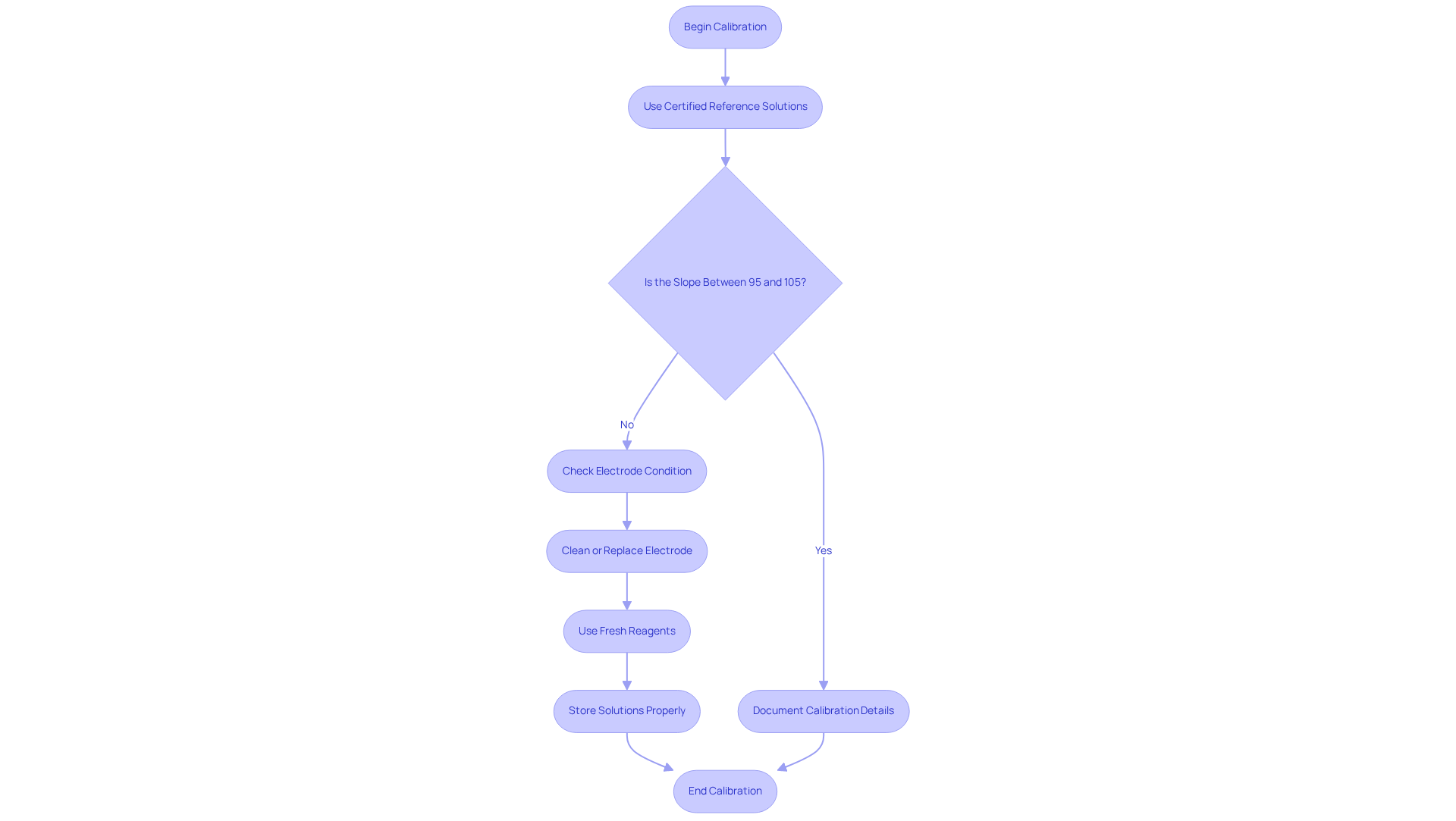
Employing pH solutions is essential for guaranteeing that pH meters deliver dependable readings. Regular calibration against certified reference pH solutions is crucial to correct any drift in readings, which can arise from factors such as electrode wear or environmental fluctuations. Laboratories should establish a regular calibration timetable, ideally documenting each calibration occurrence alongside the analyst's signature, date, time, and reference material information. This practice not only enhances precision but also ensures compliance with regulatory standards.
The during calibration should ideally fall between 95% and 105%, indicating proper electrode functionality. If the slope deviates below this range, it may signal a dirty or aging electrode, necessitating cleaning or replacement. Moreover, utilizing fresh, unexpired reagents is vital, as expired substances can lead to flawed calibration and inconsistent pH solutions. It is important to recognize that deionized water should not be used as a stabilizing agent for calibration due to its unstable pH and lack of ionic strength.
For facilities handling thick or solid samples, specialized electrodes are recommended to ensure precise readings. Real-world examples underscore the importance of maintaining proper storage conditions for pH solutions, which should be stored in tightly sealed containers at stable temperatures to prevent contamination. Adhering to these best practices not only extends the lifespan of pH sensors but also guarantees the accuracy of pH measurements across various experimental applications. As we approach 2025, compliance with updated calibration standards will be essential for laboratories striving to uphold the highest levels of accuracy and reliability in their analyses.
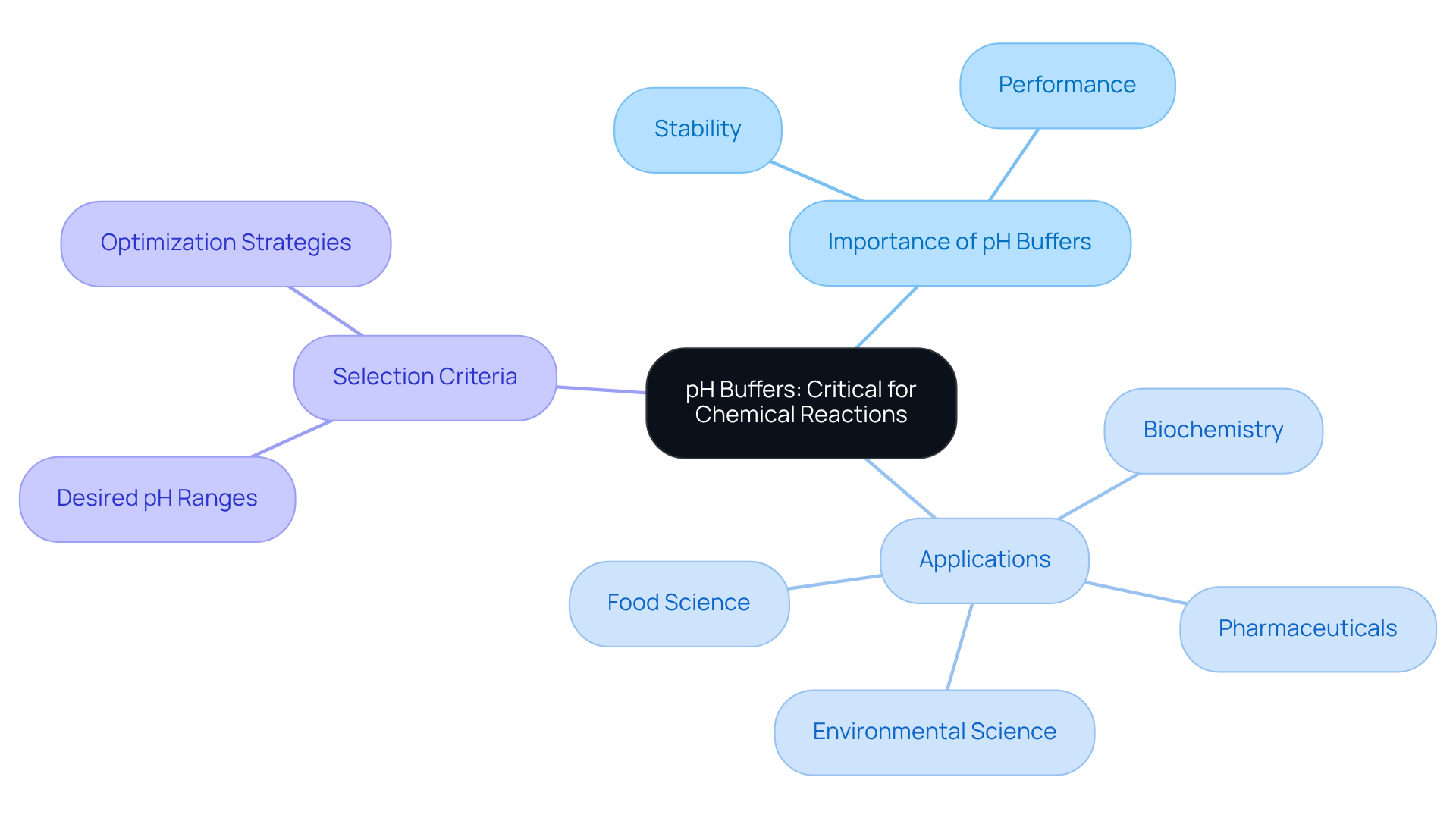
Thermo Fisher Scientific pH Buffers: Critical for Chemical Reactions
Thermo Fisher Scientific provides a diverse range of pH solutions that are essential for ensuring stable pH conditions during chemical reactions. These agents are meticulously designed to provide reliable performance across various applications, ensuring that reactions proceed as intended.
Laboratories must carefully select buffers that align with the desired pH range for their specific experiments. This strategic choice optimizes results and minimizes variability, reinforcing the critical nature of high-quality pH solutions in scientific research.
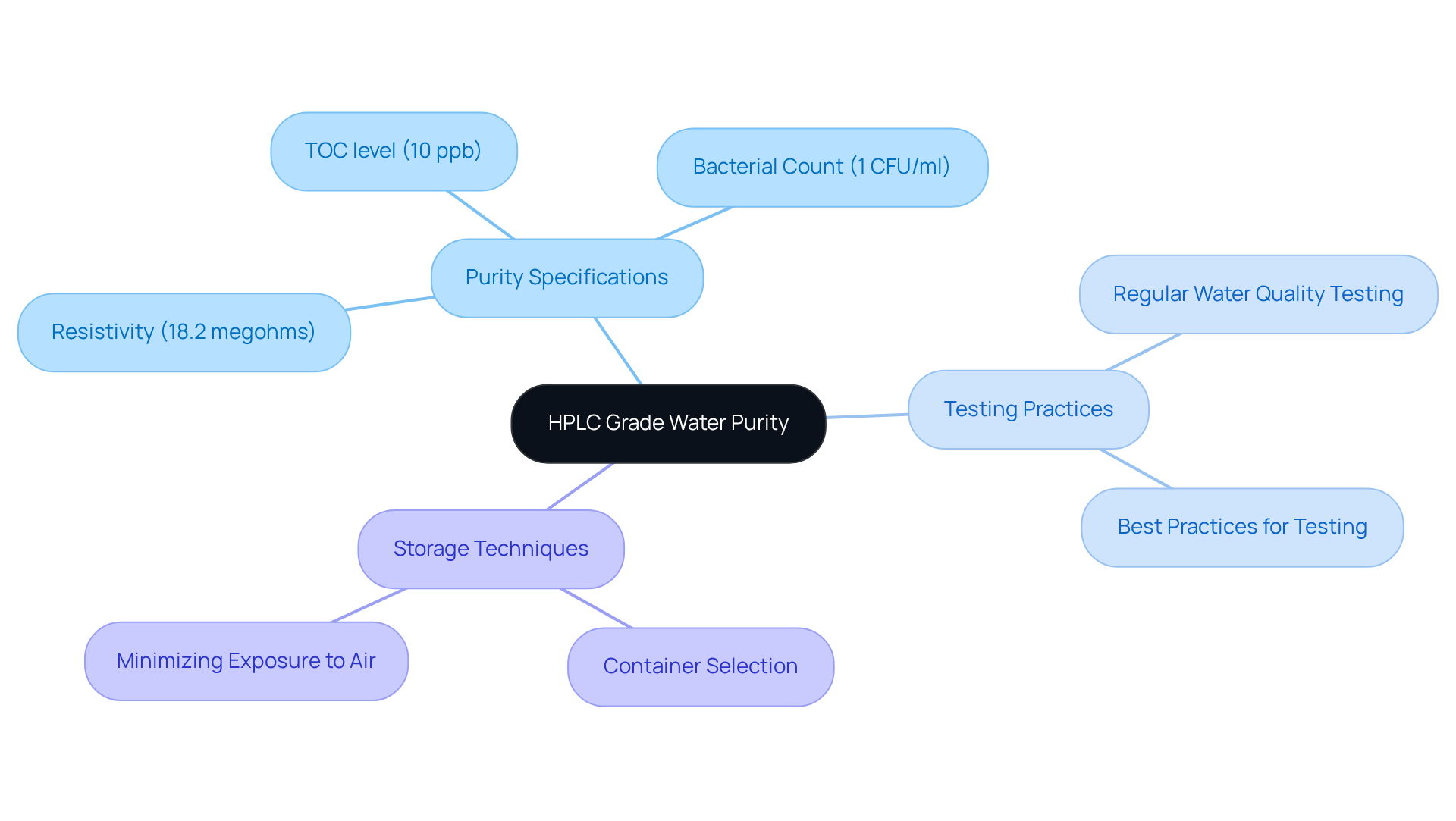
HPLC Grade Water: Purity for Accurate pH Solutions
HPLC-grade water is crucial for the preparation of accurate pH mixtures, significantly reducing the risk of contamination that can compromise results. Laboratory supervisors consistently underscore the importance of utilizing high-purity water in pH-related applications to ensure reliable readings. Studies reveal that HPLC-grade water must possess:
- A resistivity of 18.2 megohms
- A total organic carbon (TOC) level of 10 ppb
- A bacterial count of 1 CFU/ml
to guarantee minimal impurities. Even the slightest impurities can result in considerable inaccuracies in pH readings, highlighting the necessity for stringent [water quality standards](https://jmscience.com).
To maintain the integrity of pH solutions, it is imperative to , ensuring that it meets the required specifications for TOC, conductivity, and pH stability. Implementing best practices—such as using clean, sealed containers and minimizing exposure to air—further reduces contamination risks. Real-world examples demonstrate that facilities utilizing HPLC-grade water achieve more consistent and precise results, reinforcing the vital connection between water quality and measurement accuracy.
Moreover, facility managers emphasize the importance of selecting appropriate storage techniques, as leached materials from containers can jeopardize water purity. By adhering to these guidelines, laboratories can ensure that their pH solutions are both accurate and reliable, ultimately enhancing the quality of their research and outcomes.
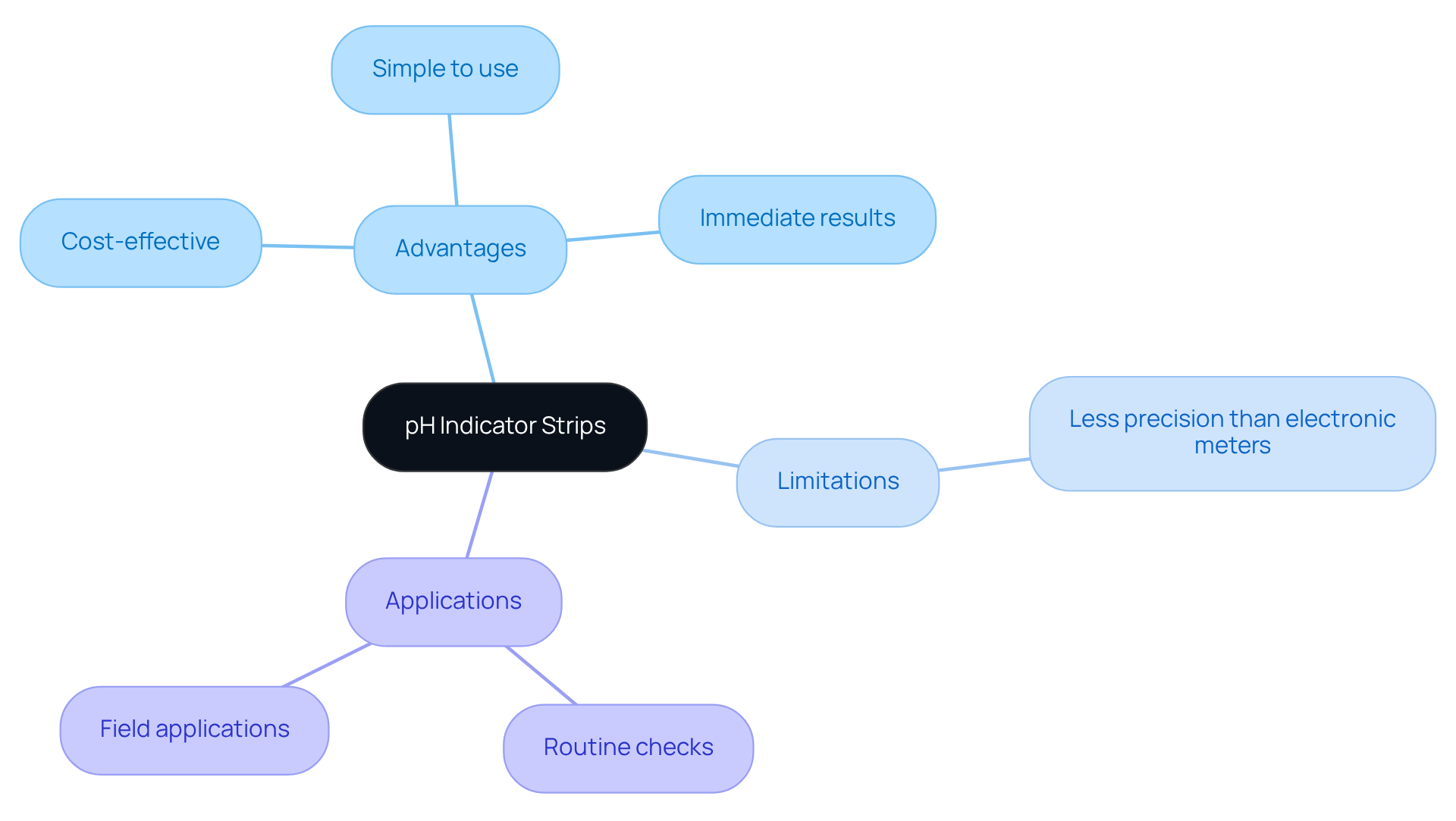
pH Indicator Strips: Visualizing pH Changes in Experiments
pH indicator strips serve as an essential tool for quickly visualizing pH changes in experiments, capturing attention with their practicality. They provide a simple and cost-effective method for assessing pH levels with pH solutions, eliminating the need for complex instrumentation. While they may lack the precision of electronic meters, their value becomes evident in routine checks and field applications, where immediate results are crucial. Thus, these strips are not merely alternatives; they are invaluable assets in any scientific setting.
Titration Solutions: Key for Determining Unknown pH Levels
Titration solutions play a pivotal role in accurately determining the pH solutions of unknown samples through systematic analysis. By meticulously adding a titrant to a sample and monitoring the resulting pH change, laboratories can confidently ascertain the pH solutions of various substances. This method is not merely a procedural step; it is a fundamental practice widely employed in pharmaceutical and chemical research, ensuring that products consistently meet specified pH solutions. The precision of titration solutions underscores their importance in maintaining the integrity of scientific outcomes and product quality.
Electronic pH Probes: Enhancing Measurement Accuracy
Electronic pH solutions are designed to enhance accuracy and sensitivity, making them the preferred choice for facilities that require precise pH measurements. These sophisticated probes leverage advanced technology to deliver real-time data, facilitating immediate adjustments and corrections.
To maintain optimal performance and reliability across various applications, regular maintenance and calibration of electronic probes are not just beneficial—they are essential. By prioritizing these practices, facilities can ensure that their pH solutions remain accurate and dependable, ultimately supporting their scientific endeavors.
Laboratory Management Software: Streamlining pH Data Analysis
Laboratory management software is crucial for optimizing the organization and analysis of pH solutions. These empower research facilities to effectively monitor data, generate comprehensive reports, and ensure compliance with regulatory standards. By integrating pH solutions into testing protocols, organizations can significantly enhance their operational efficiency and improve the accuracy of their data analysis. Notably, JM Science's specialized titrators, including Karl Fischer titrators, augment these software tools by providing precise readings that bolster data integrity. In summary, the implementation of sophisticated software solutions, combined with high-quality instruments, is essential for laboratories striving to enhance their efficiency and data accuracy in pH solutions measurement and analysis.
Conclusion
The significance of accurate pH solutions in laboratory settings cannot be overstated. By utilizing advanced technologies such as Karl Fischer titrators, buffer solutions, and reliable pH meters, laboratories can achieve the precision necessary for high-quality research and analysis. These tools not only enhance the reliability of experimental results but also ensure compliance with industry standards, thereby elevating the overall integrity of scientific work.
Throughout this article, key aspects of pH measurement have been explored, including the critical roles of calibration solutions, HPLC-grade water, and electronic pH probes. Each element contributes to the stability and accuracy of pH readings, reinforcing the necessity for meticulous preparation and ongoing maintenance in laboratory practices. Insights into buffer solutions and titration methods further highlight how these components work together to facilitate optimal conditions for various experiments.
Ultimately, the pursuit of accurate pH solutions is paramount for scientific progress. Laboratories are encouraged to adopt best practices, embrace innovative technologies, and continuously evaluate their methodologies. By doing so, they can not only enhance the quality of their results but also contribute to advancements in research across multiple fields.




